The Thiolate-Catalyzed Intermolecular Crossed Tishchenko Reaction: Highly Chemoselective Coupling of Two Different Aromatic Aldehydes†
Simon P. Curran
Centre for Synthesis and Chemical Biology, Trinity, Biomedical Sciences Institute, School of Chemistry, The University of Dublin, Trinity College, Dublin 2 (Ireland)
Search for more papers by this authorCorresponding Author
Prof. Stephen J. Connon
Centre for Synthesis and Chemical Biology, Trinity, Biomedical Sciences Institute, School of Chemistry, The University of Dublin, Trinity College, Dublin 2 (Ireland)
Centre for Synthesis and Chemical Biology, Trinity, Biomedical Sciences Institute, School of Chemistry, The University of Dublin, Trinity College, Dublin 2 (Ireland)Search for more papers by this authorSimon P. Curran
Centre for Synthesis and Chemical Biology, Trinity, Biomedical Sciences Institute, School of Chemistry, The University of Dublin, Trinity College, Dublin 2 (Ireland)
Search for more papers by this authorCorresponding Author
Prof. Stephen J. Connon
Centre for Synthesis and Chemical Biology, Trinity, Biomedical Sciences Institute, School of Chemistry, The University of Dublin, Trinity College, Dublin 2 (Ireland)
Centre for Synthesis and Chemical Biology, Trinity, Biomedical Sciences Institute, School of Chemistry, The University of Dublin, Trinity College, Dublin 2 (Ireland)Search for more papers by this authorFinancial support from the European Research Council is gratefully acknowledged.
Graphical Abstract
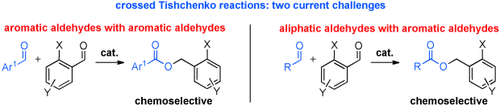
Crossed products: Ortho-substituted benzaldehydes react with other aromatic aldehydes in a highly selective, atom-economical Tishchenko disproportionation (see scheme) in the presence of a readily prepared, inexpensive thiolate-based catalyst. The methodology is of exceptionally wide scope and exhibits a high functional-group tolerance.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206343_sm_miscellaneous_information.pdf426.6 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Tischtschenko, J. Russ. Phys. Chem. 1906, 38, 355;
- 1bW. Tischtschenko, Chem. Zentralbl. 1906, 771, 1309;
- 1cL. Claisen, Ber. Dtsch. Chem. Ges. 1887, 20, 646.
10.1002/cber.188702001148 Google Scholar
- 2For recent reviews, see:
- 2aT. Seki, T. Nakajo, M. Onaka, Chem. Lett. 2006, 35, 824;
- 2bO. P. Törmäkangas, A. M. P. Koskinen, Recent Res. Dev. Org. Chem. 2001, 5, 225.
- 3S. Cannizzaro, Justus Liebigs. Ann. 1853, 88, 129.
- 4Aluminium alkoxides:
- 4aW. C. Child, H. Adkins, J. Am. Chem. Soc. 1925, 47, 798;
- 4bF. J. Villani, F. Nord, J. Am. Chem. Soc. 1947, 69, 2605;
- 4cL. Lin, A. R. Day, J. Am. Chem. Soc. 1952, 74, 5133;
- 4dT. Saegusa, T. Ueshima, J. Org. Chem. 1968, 33, 3310;
- 4eT. Ooi, T. Miura, K. Takaya, K. Maruoka, Tetrahedron Lett. 1999, 40, 7695;
- 4fT. Ooi, K. Ohmatsu, K. Sasaki, T. Miura, K. Maruoka, Tetrahedron Lett. 2003, 44, 3191;
- 4gY. Hon, C. Chang, Y. Wong, Tetrahedron Lett. 2004, 45, 3313; transition-metal-based catalysts (selected examples):
- 4hM. Yamashita, Y. Watanabe, T. Mitsudo, Y. Takegami, Bull. Chem. Soc. Jpn. 1976, 49, 3597;
- 4iT. Ito, H. Horino, Y. Koshiro, A. Yamamoto, Bull. Chem. Soc. Jpn. 1982, 55, 504;
- 4jS. H. Bergens, D. P. Fairlie, B. Bosnich, Organometallics 1990, 9, 566;
- 4kK. Morita, Y. Nishiyama, Y. Ishii, Organometallics 1993, 12, 3748;
- 4lM. Yamashita, T. Ohishi, Appl. Organomet. Chem. 1993, 7, 357;
- 4mP. Barrio, M. A. Esteruelas, E. Onate, Organometallics 2004, 23, 1340;
- 4nT. Suzuki, T. Yamada, T. Matsuo, K. Watanabe, T. Katoh, Synlett 2005, 1450;
- 4oS. Ogoshi, Y. Hoshimoto, M. Ohashi, Chem. Commun. 2010, 46, 3354; alkali metal salts:
- 4pM. M. Mojtahedi, E. Akbarzadeh, R. Sharifi, M. S. Abaee, Org. Lett. 2007, 9, 2791;
- 4qD. C. Waddell, J. Mack, Green Chem. 2009, 11, 79;
- 4rT. Werner, J. Koch, Eur. J. Org. Chem. 2010, 6904; alkali-/earth-metal-based catalysts:
- 4sM. R. Crimmin, A. G. M. Barrett, M. S. Hill, P. A. Procopiou, Org. Lett. 2007, 9, 331;
- 4tB. M. Day, N. E. Mansfield, M. P. Coles, P. B. Hitchcock, Chem. Commun. 2011, 47, 4995.
- 5For the use of boric acid as a catalyst, see: P. R. Stapp, J. Org. Chem. 1973, 38, 1433.
- 6
- 6aD. Ulrich, H. Jankowski, Chem. Tech. 1988, 40, 393;
- 6bK. Weissermel, H. J. Arpe, Industrial Organic Chemistry, 4th ed., Wiley-VCH, Weinheim, 2003;
10.1002/9783527619191 Google Scholar
- 6cA. S. Hester, K. Himmler, Ind. Eng. Chem. 1959, 51, 1424.
- 7For intramolecular crossed Tishchenko reactions, see:
- 7aJ. L. Hsu, J. M. Fang, J. Org. Chem. 2001, 66, 8573;
- 7bL. Lu, H. Y. Chang, J. M. Fang, J. Org. Chem. 1999, 64, 84.
- 8A. Chan, K. A. Scheidt, J. Am. Chem. Soc. 2006, 128, 4558.
- 9
- 9aL. Cronin, F. Manoni, C. J. O’Connor, S. J. Connon, Angew. Chem. 2010, 122, 3109;
10.1002/ange.200907167 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3045;
- 9bC. J. O’Connor, F. Manoni, S. P. Curran, S. J. Connon, New J. Chem. 2011, 35, 551.
- 10S. P. Curran, S. J. Connon, Org. Lett. 2012, 14, 1074.
- 11Y. Hoshimoto, M. Ohashi, S. Ogoshi, J. Am. Chem. Soc. 2011, 133, 4668.
- 12For a Highlight article, see: W. I. Dzik, L. J. Gooßen, Angew. Chem. 2011, 123, 11241;
10.1002/ange.201105423 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11047.
- 13For example, Ogoshi and co-workers (Ref. [11]) attempted both these types of reactions under conditions previously found to be beneficial to selective aliphatic–aromatic Tishchenko chemistry. However, reactions involving either two different aliphatic or two different aromatic aldehydes were found to be unselective.
- 14Eisen had already shown that actinide-complex-catalyzed crossed Tishchenko reactions involving aromatic aldehydes of similar electronic characteristics were unselective: T. Andrea, E. Barnea, M. S. Eisen, J. Am. Chem. Soc. 2008, 130, 2454.
- 15The thioester- and alkoxide-derived alcohol can be observed during the reaction by 1H NMR spectroscopy and both have been shown to serve as competent intermediates in the cycle. See Ref. [9a].
- 16
- 16aJ. M. Berg, J. L. Tymoczko, L. Stryer, Biochemistry, 5th ed., Freeman, New York, 2002;
- 16bA. Soukri, A. Mougin, C. Corbier, A. Wonacott, C. Branlant, G. Branlant, Biochemistry 1989, 28, 2586;
- 16cK. D’Ambrosio, A. Pailot, F. Talfournier, C. Didierjean, E. Benedetti, A. Aubry, G. Branlant, C. Corbier, Biochemistry 2006, 45, 2978.
- 17Our group in collaboration with Zeitler and co-workers employed a similar strategy to develop highly selective (mechanistically distinct) crossed acyloin reactions. S. E. O’Toole, C. A. Rose, S. Gundala, K. Zeitler, S. J. Connon, J. Org. Chem. 2011, 76, 347.
- 18It is tempting to ascribe this observation to reduced conjugation between the carbonyl group and the electron-rich aromatic ring, however, attempts to rationalize this observation are perhaps best left until after the emergence of a clearer mechanistic picture.
- 19Naturally, the chelating substituent would also stabilize the hemithioacetal conjugate base. However, as we have pointed out, steric effects are likely to make more of a contribution to the stability (or lack thereof) of that species than to the hydride-transfer adduct.
- 20For example, Nicolaou et al. have employed benzyl benzoates containing o-bromo substituents (similar to 51) as key components in the total synthesis of balanol (and analogues thereof): K. C. Nicolaou, K. Koide, M. E. Bunnage, Chem. Eur. J. 1995, 1, 454.
- 21The coupled product 47 could not be cleanly isolated from the crude material. Therefore the yield of this compound was determined unambiguously by 1H NMR spectroscopy using styrene as an internal standard. The yields given for 48, 49, and 49 a are those of isolated products. For a review of the hydrogenolysis of sulfur-containing compounds using Raney nickel, see: H. Hauptmann, W. F. Walter, Chem. Rev. 1962, 62, 347.
- 22Aliphatic aldehydes containing α-unbranched aldehydes undergo preferential aldol condensation under these conditions.