Transition Metal Complexes of Anionic N-Heterocyclic Dicarbene Ligands†
Rebecca A. Musgrave
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorRobert S. P. Turbervill
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorMark Irwin
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorCorresponding Author
Dr. Jose M. Goicoechea
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspxSearch for more papers by this authorRebecca A. Musgrave
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorRobert S. P. Turbervill
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorMark Irwin
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Search for more papers by this authorCorresponding Author
Dr. Jose M. Goicoechea
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspx
Department of Chemistry, University of Oxford, Chemistry Research Laboratory, Mansfield Road, Oxford, OX1 3TA (UK) http://research.chem.ox.ac.uk/jose-goicoechea.aspxSearch for more papers by this authorWe thank the EPSRC and the University of Oxford for financial support of this research (DTA studentships to R.S.P.T. and M.I.) and the University of Oxford for access to Chemical Crystallography and Oxford Supercomputing Centre facilities. We also thank Elemental Microanalysis Ltd. (Devon) for performing the elemental analyses.
Graphical Abstract
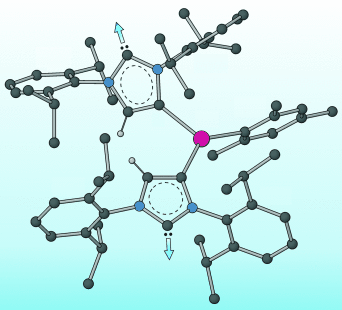
A tale of two carbenes: Reaction of :C[N(2,6-iPr2C6H3)CH]2 (IPr) with Mn3(mes)6 (mes=2,4,6-trimethylphenyl) yielded the trigonal planar complex [Mn(IPr)(mes)2]. Reduction of this species with potassium/graphite in THF afforded the polymeric dicarbene-bridged species K[{:C[N(2,6-iPr2C6H3)]2(CH)C}2Mn(mes)(thf)]⋅THF (see picture). The anionic moiety in this complex is the first reported example of a transition metal complex containing an N-heterocyclic dicarbene ligand. Gray C, blue N, red Mn.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206100_sm_miscellaneous_information.pdf1.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aH.-W. Wanzlick, H.-J. Schönherr, Angew. Chem. 1968, 80, 154;
10.1002/ange.19680800411 Google ScholarAngew. Chem. Int. Ed. Engl. 1968, 7, 141;
- 1bK. Öfele, J. Organomet. Chem. 1968, 12, P 42.
- 2A. J. Arduengo III, R. L. Harlow, M. Kline, J. Am. Chem. Soc. 1991, 113, 361.
- 3
- 3a Carbene Chemistry: From Fleeting Intermediates to Powerful Reagents (Ed.: ), Marcel Dekker, New York, 2002;
- 3bD. Bourissou, O. Guerret, F. P. Gabbaï, G. Bertrand, Chem. Rev. 2000, 100, 39;
- 3c N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools (RSC Catalysis Series) (Ed.: ), RSC Publishing, Cambridge 2010.
- 4For recent reviews, see:
- 4aL. Mercs, M. Albrecht, Chem. Soc. Rev. 2010, 39, 1903;
- 4bS. Díez-González, N. Marion, S. P. Nolan, Chem. Rev. 2009, 109, 3612;
- 4cH. Jacobsen, A. Correa, A. Poater, C. Costabile, L. Cavallo, Coord. Chem. Rev. 2009, 253, 687;
- 4dF. E. Hahn, M. Jahnke, Angew. Chem. 2008, 120, 3166; Angew. Chem. Int. Ed. 2008, 47, 3122;
- 4eS. Díez-González, S. P. Nolan, Coord. Chem. Rev. 2007, 251, 874;
- 4f N-Heterocyclic Carbenes in Synthesis (Ed.: ), Wiley-VCH, Weinheim, 2006;
- 4g N-Heterocyclic Carbenes in Transition Metal Catalysis (Topics in Organometallic Chemistry 21) (Ed.: ), Springer, Berlin, 2006;
- 4h N-Heterocyclic Carbenes in Transition Metal Catalysis and Organocatalysis (Catalysis by Metal Complexes 32) (Ed.: ), Springer, Dordrecht, 2011.
- 5Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schafer III; P. v. R. Schleyer, G. H. Robinson, Science 2008, 321, 1069; P. v. R. Schleyer, G. H. Robinson, Science 2008, 321, 1069.
- 6A. Sidiropoulos, C. Jones, A. Stasch, S. Klein, G. Frenking, Angew. Chem. 2009, 121, 9881;
10.1002/ange.200905495 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 9701.
- 7aY. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2008, 130, 14970;
- 7bO. Back, G. Kuchenbeiser, B. Donnadieu, G. Bertrand, Angew. Chem. 2009, 121, 5638;
10.1002/ange.200902344 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5530.
- 8M. Y. Abraham, Y. Wang, Y. Xie, P. Wei, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, Chem. Eur. J. 2010, 16, 432.
- 9O. Back, B. Donnadieu, P. Parameswaran, G. Frenking, G. Bertrand, Nat. Chem. 2010, 2, 369.
- 10S. Gründemann, A. Kovacevic, M. Albrecht, J. W. Faller, R. H. Crabtree, Chem. Commun. 2001, 2274.
- 11
- 11aO. Schuster, L. Yang, H. G. Raubenheimer, M. Albrecht, Chem. Rev. 2009, 109, 3445;
- 11bP. L. Arnold, S. Pearson, Coord. Chem. Rev. 2007, 251, 596;
- 11cM. Albrecht, Chem. Commun. 2008, 3601.
- 12E. Aldeco-Perez, A. J. Rosenthal, B. Donnadieu, P. Parameswaran, G. Frenking, G. Bertrand, Science 2009, 326, 556.
- 13G. Guisado-Barrios, J. Bouffard, B. Donnadieu, G. Bertrand, Angew. Chem. 2010, 122, 4869;
10.1002/ange.201001864 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4759.
- 14
- 14aY. Wang, Y. Xie, M. Y. Abraham, P. Wei, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, J. Am. Chem. Soc. 2010, 132, 14370;
- 14bY. Wang, Y. Xie, M. Y. Abraham, P. Wei, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, Organometallics 2011, 30, 1303;
- 14cY. Wang, M. Y. Abraham, R. J. Gilliard, Jr., P. Wei, J. C. Smith, G. H. Robinson, Organometallics 2012, 31, 791.
- 15S. Kronig, E. Theuergarten, C. G. Daniliuc, P. G. Jones, M. Tamm, Angew. Chem. 2012, 124, 3294;
10.1002/ange.201108813 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3240.
- 16P. L. Arnold, S. T. Liddle, Organometallics 2006, 25, 1485.
- 17
- 17aO. Guerret, S. Sole, H. Gornitzka, M. Teichert, G. Trinquier, G. Bertrand, J. Am. Chem. Soc. 1997, 119, 6668;
- 17bO. Guerret, S. Sole, H. Gornitzka, G. Trinquier, G. Bertrand, J. Organomet. Chem. 2000, 600, 112.
- 18See the Supporting Information for full synthetic and crystallographic details.
- 19A. R. Kennedy, R. E. Mulvey, J. Klett, S. D. Robertson, Eur. J. Inorg. Chem. 2011, 4675.