Enantioselective Rhodium-Catalyzed Synthesis of Branched Allylic Amines by Intermolecular Hydroamination of Terminal Allenes†
Dr. Michael L. Cooke
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Search for more papers by this authorKun Xu
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernhard Breit
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)Search for more papers by this authorDr. Michael L. Cooke
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Search for more papers by this authorKun Xu
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Bernhard Breit
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)
Institut für Organische Chemie und Biochemie, Freiburg Institute for Advanced Studies (FRIAS), Albert-Ludwigs-Universität Freiburg, Albertstrasse 21, 79104 Freiburg im Breisgau (Germany)Search for more papers by this authorThis work was supported by the DFG, the International Research Training Group “Catalysts and Catalytic Reactions for Organic Synthesis” (IRTG 1038), the Fonds der Chemischen Industrie, and the Krupp Foundation. We thank Umicore, BASF, and Wacker for generous gifts of chemicals.
Graphical Abstract
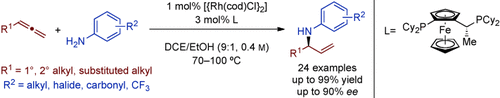
Branching out: The rhodium-catalyzed enantioselective hydroamination of monosubstituted allenes with anilines permits the atom-economic synthesis of valuable branched allylic amines. In contrast to previous linear selective allene hydroaminations, a RhI/Josiphos catalyst system (see scheme; cod=1,5-cyclooctadiene, DCE=1,2-dichloroethane) allows branched allylic amines to be obtained with perfect regioselectivity, high yield, and good enantioselectivity.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206594_sm_miscellaneous_information.pdf3.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For reviews concerning the synthesis of α-chiral allylic amines, see:
- 1aJ. F. Hartwig, L. M. Stanley, Acc. Chem. Res. 2010, 43, 1461–1475;
- 1bB. M. Trost, T. Zhang, J. D. Sieber, Chem. Sci. 2010, 1, 427–440;
- 1cZ. Lu, S. Ma, Angew. Chem. 2008, 120, 264–303; Angew. Chem. Int. Ed. 2008, 47, 258–297;
- 1dE. Skucas, M.-Y. Ngai, V. Komanduri, M. J. Krische, Acc. Chem. Res. 2007, 40, 1394–1401;
- 1eG. Helmchen, A. Dahnz, P. Dübon, M. Schelwies, R. Weihofen, Chem. Commun. 2007, 675–691;
- 1fL. E. Overman, N. E. Carpenter, Organic Reactions, Wiley, Hoboken, 2005, pp. 1–107;
- 1gB. M. Trost, M. L. Crawley, Chem. Rev. 2003, 103, 2921–2944;
- 1hS. Kobayashi, H. Ishitani, Chem. Rev. 1999, 99, 1069–1094;
- 1iM. Johannsen, K. A. Jørgensen, Chem. Rev. 1998, 98, 1689–1708;
- 1jR. Bloch, Chem. Rev. 1998, 98, 1407–1438.
- 2For a recent example of the allylic substituion of unactivated allylic alcohols, see: M. Lafrance, M. Roggen, E. M. Carreira, Angew. Chem. 2012, 124, 3527–3530;
10.1002/ange.201108287 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3470–3473.
- 3For an example of an imine vinylation which avoids stoichiometric organometallics, see: M.-Y. Ngai, A. Barchuk, M. J. Krische, J. Am. Chem. Soc. 2007, 129, 12644–12645.
- 4For reviews on catalytic hydroamination, see:
- 4aS. R. Chemler, Org. Biomol. Chem. 2009, 7, 3009–3019;
- 4bT. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo, M. Tada, Chem. Rev. 2008, 108, 3795–3892;
- 4cI. Aillaud, J. Collin, J. Hannedouche, E. Schulz, Dalton Trans. 2007, 5105–5118;
- 4dR. A. Widenhoefer, X. Han, Eur. J. Org. Chem. 2006, 4555–4563;
- 4eK. C. Hultzsch, Adv. Synth. Catal. 2005, 347, 367–391;
- 4fM. Beller, J. Seayad, A. Tillack, H. Jiao, Angew. Chem. 2004, 116, 3448–3479;
10.1002/ange.200300616 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 3368–3398.
- 5For examples of enantioselective intermolecular hydroamination, see:
- 5aC. S. Sevov, J. Zhou, J. F. Hartwig, J. Am. Chem. Soc. 2012, 134, 11960–11963;
- 5bS. Pan, K. Endo, T. Shibata, Org. Lett. 2012, 14, 780–783;
- 5cM. J. MacDonald, D. J. Schipper, P. J. Ng, J. Moran, A. M. Beauchemin, J. Am. Chem. Soc. 2011, 133, 20100–20103;
- 5dA. L. Reznichenko, H. N. Nguyen, K. C. Hultzsch, Angew. Chem. 2010, 122, 9168–9171;
10.1002/ange.201004570 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 8984–8987;
- 5eZ. Zhang, S. D. Lee, R. A. Widenhoefer, J. Am. Chem. Soc. 2009, 131, 5372–5373;
- 5fJ. Zhou, J. F. Hartwig, J. Am. Chem. Soc. 2008, 130, 12220–12221;
- 5gA. Hu, M. Ogasawara, T. Sakamoto, A. Okada, K. Nakajima, T. Takahashi, W. Lin, Adv. Synth. Catal. 2006, 348, 2051–2056;
- 5hK. Li, P. N. Horton, M. B. Hursthouse, K. K. Hii, J. Organomet. Chem. 2003, 665, 250–257;
- 5iM. Utsunomiya, J. F. Hartwig, J. Am. Chem. Soc. 2003, 125, 14286–14287;
- 5jO. Löber, M. Kawatsura, J. F. Hartwig, J. Am. Chem. Soc. 2001, 123, 4366–4367;
- 5kM. Kawatsura, J. F. Hartwig, J. Am. Chem. Soc. 2000, 122, 9546–9547;
- 5lR. Dorta, P. Egli, F. Zürcher, A. Togni, J. Am. Chem. Soc. 1997, 119, 10857–10858.
- 6For examples of the enantioselective intramolecular hydroamination of allenes, see:
- 6aH. Li, S. Du Lee, R. A. Widenhoefer, J. Organomet. Chem. 2011, 696, 316–320;
- 6bR. L. LaLonde, Z. J. Wang, M. Mba, A. D. Lackner, F. D. Toste, Angew. Chem. 2010, 122, 608–611;
10.1002/ange.200905000 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 598–601;
- 6cG. L. Hamilton, E. J. Kang, M. Mba, F. D. Toste, Science 2007, 317, 496–499;
- 6dZ. Zhang, C. F. Bender, R. A. Widenhoefer, Org. Lett. 2007, 9, 2887–2889;
- 6eZ. Zhang, C. F. Bender, R. A. Widenhoefer, J. Am. Chem. Soc. 2007, 129, 14148–14149;
- 6fR. L. LaLonde, B. D. Sherry, E. J. Kang, F. D. Toste, J. Am. Chem. Soc. 2007, 129, 2452–2453;
- 6gJ. M. Hoover, J. R. Petersen, J. H. Pikul, A. R. Johnson, Organometallics 2004, 23, 4614–4620.
- 7For the enantioselective intramolecular hydroamination of alkynes affording similar products, see:
- 7aM. Narsireddy, Y. Yamamoto, J. Org. Chem. 2008, 73, 9698–9709;
- 7bL. M. Lutete, I. Kadota, Y. Yamamoto, J. Am. Chem. Soc. 2004, 126, 1622–1623.
- 8K. L. Butler, M. Tragni, R. A. Widenhoefer, Angew. Chem. 2012, 124, 5265–5268; Angew. Chem. Int. Ed. 2012, 51, 5175–5178.
- 9For a report on the enantiospecific intermolecular hydroamination of chiral allenes, see: N. Nishina, Y. Yamamoto, Angew. Chem. 2006, 118, 3392–3395;
10.1002/ange.200600331 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3314–3317.
- 10
- 10aA. N. Duncan, R. A. Widenhoefer, Synlett 2010, 419–422;
- 10bK. L. Toups, R. A. Widenhoefer, Chem. Commun. 2010, 46, 1712–1714;
- 10cX. Zeng, M. Soleilhavoup, G. Bertrand, Org. Lett. 2009, 11, 3166–3169;
- 10dN. Nishina, Y. Yamamoto, Tetrahedron 2009, 65, 1799–1808;
- 10eN. Nishina, Y. Yamamoto, Synlett 2007, 1767–1770;
- 10fR. O. Ayinla, L. L. Schafer, Inorg. Chim. Acta 2006, 359, 3097–3102;
- 10gJ. S. Johnson, R. G. Bergman, J. Am. Chem. Soc. 2001, 123, 2923–2924;
- 10hM. Al-Masum, M. Meguro, Y. Yamamoto, Tetrahedron Lett. 1997, 38, 6071–6074;
- 10iL. Besson, J. Goré, B. Cazes, Tetrahedron Lett. 1995, 36, 3857–3860.
- 11
- 11aA. Lumbroso, N. Abermil, B. Breit, Chem. Sci. 2012, 3, 789–793;
- 11bP. Koschker, A. Lumbroso, B. Breit, J. Am. Chem. Soc. 2011, 133, 20746–20749;
- 11cA. Lumbroso, P. Koschker, N. R. Vautravers, B. Breit, J. Am. Chem. Soc. 2011, 133, 2386–2389;
- 11dA. Lumbroso, N. R. Vautravers, B. Breit, Org. Lett. 2010, 12, 5498–5501.
- 12For the rhodium-catalyzed hydroamination of norbornene with aniline, see:
- 12aJ.-J. Brunet, D. Neibecker, K. Philippot, J. Chem. Soc. Chem. Commun. 1992, 1215–1216;
- 12bJ.-J. Brunet, G. Commenges, D. Neibecker, K. Philippot, J. Organomet. Chem. 1994, 469, 221–228.
- 13L. E. Overman, C. E. Owen, M. M. Pavan, C. J. Richards, Org. Lett. 2003, 5, 1809–1812.
- 14No deuterium incorporation was observed in the reaction between cyclohexylallene with aniline in DCE/CD3OH, which suggests that a mechanism involving hydride transfer from the alcohol is unlikely.
- 15A. L. Casalnuovo, J. C. Calabrese, D. Milstein, J. Am. Chem. Soc. 1988, 110, 6738–6744.
- 16
- 16aD. C. Vrieze, G. S. Hoge, P. Z. Hoerter, J. T. Van Haitsma, B. M. Samas, Org. Lett. 2009, 11, 3140–3142;
- 16bT. Hayashi, A. Okada, T. Suzuka, M. Kawatsura, Org. Lett. 2003, 5, 1713–1715;
- 16cP. A. Evans, D. K. Leahy, J. Am. Chem. Soc. 2002, 124, 7882–7883;
- 16dP. A. Evans, J. E. Robinson, J. Am. Chem. Soc. 2001, 123, 4609–4610;
- 16eP. A. Evans, L. J. Kennedy, J. Am. Chem. Soc. 2001, 123, 1234–1235;
- 16fP. A. Evans, D. K. Leahy, J. Am. Chem. Soc. 2000, 122, 5012–5013;
- 16gP. A. Evans, J. E. Robinson, J. D. Nelson, J. Am. Chem. Soc. 1999, 121, 6761–6762;
- 16hP. A. Evans, J. D. Nelson, J. Am. Chem. Soc. 1998, 120, 5581–5582.