Photoisomerization of 1,2-Dihydro-1,2-Azaborine: A Matrix Isolation Study†
Dr. Sarah A. Brough
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany)
Search for more papers by this authorAshley N. Lamm
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorProf. Dr. Shih-Yuan Liu
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger F. Bettinger
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany)
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany)Search for more papers by this authorDr. Sarah A. Brough
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany)
Search for more papers by this authorAshley N. Lamm
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorProf. Dr. Shih-Yuan Liu
Department of Chemistry, University of Oregon, Eugene, OR 97403-1253 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Holger F. Bettinger
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany)
Institut für Organische Chemie, Universität Tübingen, Auf der Morgenstelle 18, 72076 Tübingen (Germany)Search for more papers by this authorSupport for this research has been provided by the Deutsche Forschungsgemeinschaft, the Fonds der chemischen Industrie, the National Science Foundation (Grant DGE-0742540; A.N.L), and the National Institutes of Health (Grant R01-GM094541).
Graphical Abstract
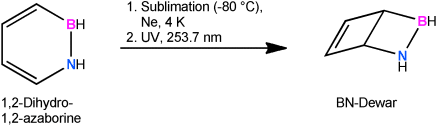
Closing the loop: Photoisomerization of 1,2-dihydro-1,2-azaborine in neon, argon, or xenon at 4 K with UV light (253.7 nm) as part of a matrix isolation study led to the Dewar form as the only photoproduct, in agreement with the vibrational spectra computed for possible isomers of 1,2-dihydro-1,2-azaborine.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201203546_sm_miscellaneous_information.pdf1.9 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Hatakeyama, S. Hashimoto, S. Seki, M. Nakamura, J. Am. Chem. Soc. 2011, 133, 18614–18617;
- 1bC. A. Jaska, D. J. H. Emslie, M. J. D. Bosdet, W. E. Piers, T. S. Sorensen, M. Parvez, J. Am. Chem. Soc. 2006, 128, 10885–10896;
- 1cS. M. Mansell, N. C. Norman, C. A. Russel, Dalton Trans. 2010, 39, 5084–5086.
- 2For reviews see:
- 2aP. G. Campbell, A. J. V. Marwitz, S.-Y. Liu, Angew. Chem. 2012, 124, 6178–6197;
10.1002/ange.201200063 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 6074–6092;
- 2bM. J. D. Bosdet, W. E. Piers, Can. J. Chem. 2008, 86, 8–29;
- 2cZ. Liu, T. B. Marder, Angew. Chem. 2008, 120, 248–250; Angew. Chem. Int. Ed. 2008, 47, 242–244.
- 3
- 3aD. G. White, J. Am. Chem. Soc. 1963, 85, 3634–3636;
- 3bM. J. S. Dewar, P. A. Marr, J. Am. Chem. Soc. 1962, 84, 3782.
- 4
- 4aA. J. Ashe III, X. Fang, Org. Lett. 2000, 2, 2089–2091;
- 4bA. J. Ashe III, X. Fang, X. Fang, J. W. Kampf, Organometallics 2001, 20, 5413–5418.
- 5
- 5aJ. Pan, J. W. Kampf, A. J. Ashe III, Organometallics 2004, 23, 5626–5629;
- 5bJ. Pan, J. W. Kampf, A. J. Ashe III, Organometallics 2006, 25, 197–202;
- 5cJ. Pan, J. W. Kampf, A. J. Ashe, Organometallics 2008, 27, 1345–1347;
- 5dJ. Pan, J. W. Kampf, A. J. Ashe III, J. Organomet. Chem. 2009, 694, 1036–1040.
- 6J. Pan, J. W. Kampf, A. J. Ashe, Org. Lett. 2007, 9, 679–681.
- 7
- 7aE. R. Abbey, L. N. Zakharov, S.-Y. Liu, J. Am. Chem. Soc. 2008, 130, 7250–7252;
- 7bP. J. Silva, M. J. Ramos, J. Org. Chem. 2009, 74, 6120–6129;
- 7cC. Tanjaroon, A. Daly, A. J. V. Marwitz, S.-Y. Liu, S. Kukolich, J. Chem. Phys. 2009, 131, 224312;
- 7dJ. E. Del Bene, M. Yáñez, I. Alkorta, J. Elguero, J. Chem. Theory Comput. 2009, 5, 2239–2247;
- 7eR. Carion, V. Liégeois, B. Champagne, D. Bonifazi, S. Pelloni, P. Lazzeretti, J. Phys. Chem. Lett. 2010, 1, 1563–1568;
- 7fM. Kranz, T. Clark, J. Org. Chem. 1992, 57, 5492–5500.
- 8P. G. Campbell, L. N. Zakharov, D. J. Grant, D. A. Dixon, S.-Y. Liu, J. Am. Chem. Soc. 2010, 132, 3289–3291.
- 9
- 9aA. J. V. Marwitz, M. H. Matus, L. N. Zakharov, D. A. Dixon, S.-Y. Liu, Angew. Chem. 2009, 121, 991–995;
10.1002/ange.200805554 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 973–977;
- 9bA. Chrostowska, S. Xu, A. N. Lamm, A. Maziere, C. D. Weber, A. Dargelos, P. Baylere, A. Graciaa, S.-Y. Liu, J. Am. Chem. Soc. 2012, 134, 10279–10285.
- 10P. G. Campbell, E. R. Abbey, D. Neiner, D. J. Grant, D. A. Dixon, S.-Y. Liu, J. Am. Chem. Soc. 2010, 132, 18048.
- 11A. N. Lamm, E. B. Garner III, D. A. Dixon, S.-Y. Liu, Angew. Chem. 2011, 123, 8307–8310;
10.1002/ange.201103192 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 8157–8160.
- 12A. J. V. Marwitz, J. T. Jenkins, L. V. Zakharov, S.-Y. Liu, Angew. Chem. 2010, 122, 7606–7609;
10.1002/ange.201004084 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 7444–7447.
- 13
- 13aP. J. Harman, J. E. Kent, M. F. O’Dwyer, D. W. T. Griffith, J. Phys. Chem. 1981, 85, 2731–2733;
- 13bW. A. Noyes, Jr., K. E. Al-Ani, Chem. Rev. 1974, 74, 29–43;
- 13c A. Gilbert in Photochemistry, Vol. 36 (Ed.: ), RSC Publishing, Cambridge, 2007, pp. 91–132, and earlier volumes.
- 14
- 14aE. E. Van Tamelen, S. P. Pappas, J. Am. Chem. Soc. 1963, 85, 3297—3298;
- 14bK. E. Wilzbach, J. S. Ritscher, L. Kaplan, J. Am. Chem. Soc. 1967, 89, 1031–1032;
- 14c T. J. Katz, N. Acton, J. Am. Chem. Soc. 1973, 95, 2738–2739;
- 14dW. E. Billups, M. M. Haley, Angew. Chem. 1989, 101, 1735–1737; Angew. Chem. Int. Ed. Engl. 1989, 28, 1711–1712.
- 15
- 15aU. D. Priyakumar, T. C. Dinadayalane, G. N. Sastry, New J. Chem. 2002, 26, 347–353;
- 15bZ. Li, D. W. Rogers, F. J. McLafferty, M. Mandziuk, A. V. Podosenin, J. Phys. Chem. A 1999, 103, 426–430;
- 15cJ. M. Schulman, R. L. Disch, J. Am. Chem. Soc. 1985, 107, 5059–5061;
- 15dM. D. Newton, J. M. Schulman, M. M. Manus, J. Am. Chem. Soc. 1974, 96, 17–23.
- 16H. R. Ward, J. S. Wishnok, J. Am. Chem. Soc. 1968, 90, 1085–1086.
- 17L. Kaplan, K. E. Wilzbach, J. Am. Chem. Soc. 1967, 89, 1030–1031.
- 18D. E. Johnstone, J. R. Sodeau, J. Phys. Chem. 1991, 95, 165–169.
- 19K. E. Wilzbach, D. J. Rausch, J. Am. Chem. Soc. 1970, 92, 2178–2179.
- 20S. Kudoh, M. Takayanagi, M. Nakata, J. Photochem. Photobiol. A 1999, 123, 25–30.
- 21The neon data are discussed herein as they are of higher quality. Experiments performed in argon or xenon gave similar results; spectra and band tables can be found in the Supporting Information.
- 22E. A. McNeill, F. R. Scholer, J. Mol. Struct. 1976, 31, 65–72.
- 23P. Paetzold, Pure Appl. Chem. 1991, 63, 345–350.
- 24
- 24aE. E. Van Tamelen, S. P. Pappas, J. Am. Chem. Soc. 1962, 84, 3789–3791;
- 24bE. E. Van Tamelen, Angew. Chem. 1965, 77, 759–767; Angew. Chem. Int. Ed. Engl. 1965, 4, 738–745;
- 24cE. E. Van Tamelen, S. P. Pappas, K. L. Kirk, J. Am. Chem. Soc. 1971, 93, 6092–6101.
- 25
- 25aP. Paetzold, C. von Plotho, G. Schmid, R. Boese, Z. Naturforsch. B 1984, 39, 1069–1075;
- 25bH. A. Steuer, A. Meller, G. Elter, J. Organomet. Chem. 1985, 295, 1–6;
- 25cP. Paetzold, J. Kiesgen, K. Krahe, H. U. Meier, R. Boese, Z. Naturforsch. B 1991, 46, 853–860;
- 25dJ. Münster, P. Paetzold, E. Schröder, H. Schwan, T. von Bennigsen-Mackiewicz, Z. Anorg. Allg. Chem. 2004, 630, 2641–2651.
- 26For reviews see:
- 26aP. Paetzold, Adv. Inorg. Chem. 1987, 31, 123–170;
- 26bP. Paetzold, Phosphorus Sulfur Silicon Relat. Elem. 1994, 93–94, 39–50.
- 27I. R. Dunkin, Matrix-Isolation Techniques, Oxford University Press, New York, 1998.
- 28
- 28aA. D. Becke, J. Chem. Phys. 1993, 98, 5648—5652;
- 28bC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785—789;
- 28cFrisch et al., GAUSSIAN 09, Revision C.01, Gaussian Inc., Wallingford, CT (USA), 2010.
- 29
- 29aK. Raghavachari, G. W. Trucks, J. A. Pople, M. Head-Gordon, Chem. Phys. Lett. 1989, 157, 479–483;
- 29bR. J. Bartlett, J. D. Watts, S. A. Kucharski, J. Noga, Chem. Phys. Lett. 1990, 165, 513–522;
- 29cG. E. Scuseria, J. Chem. Phys. 1991, 94, 442–447.
- 30T. H. Dunning, J. Chem. Phys. 1989, 90, 1007–1023.
- 31J. F. Stanton et al., CFOUR, Coupled-Cluster techniques for Computational Chemistry; for the current version, see http://www.cfour.de.