Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: Organocatalysis by Neutral Multidentate Halogen-Bond Donors (Angew. Chem. Int. Ed. 27/2013)
- Page: 6787
- First Published: 05 June 2013

Neutral multidentate halogen-bond donors (halogen-based Lewis acids) can be used as organocatalysts in a halide abstraction reaction. In their Communication on page 7028 ff., S. M. Huber et al. describe the halogen-bond-catalyzed reaction of 1-chloroisochroman with ketene silyl acetals. The organocatalytic activity crucially depends on the iodine substituents of the halogen-bond donors, and hidden acid catalysts can be ruled out with high probability.
Inside Cover: Stereoselective Rearrangements with Chiral Hypervalent Iodine Reagents (Angew. Chem. Int. Ed. 27/2013)
- Page: 6788
- First Published: 31 May 2013

Iodine has made a long journey from being a table salt additive to the key element in efficient chiral reagents. In their Communication on page 7018 ff. T. Wirth and co-workers describe the utilization of such reagents in stereoselective oxidative rearrangements of alkenes. For the first time, rearrangements to α-arylated ketones can be performed in high enantioselectivities using chiral hypervalent iodine reagents based on lactic acid.
Inside Back Cover: Ultrafast Solvent-Assisted Electronic Level Crossing in 1-Naphthol (Angew. Chem. Int. Ed. 27/2013)
- Page: 7043
- First Published: 06 June 2013

Photoacidic properties of the prototypical molecule 1-naphthol have long been claimed to involve an inversion between the two first-excited singlet states, controlled by the polarity of the solvent. In their Communication on page 6871 ff., E. T. J. Nibbering, M. Chergui, et al. address the non-adiabatic excited-state dynamics of 1-naphthol with femtosecond time resolution and report the first direct experimental observation of the ultrafast level inversion.
Back Cover: Mix to Validate: A Facile, Reversible PEGylation for Fast Screening of Potential Therapeutic Proteins In Vivo (Angew. Chem. Int. Ed. 27/2013)
- Page: 7044
- First Published: 06 June 2013

Increasing the half-life of therapeutic proteins in a specific, fast, and reliable way could greatly benefit the discovery of novel protein drugs. In their Communication on page 6880 ff., K. C. Lee, S. Lee, and co-workers conjugate Ni-NTA to poly(ethylene glycol) and then label oligohistidine-tagged therapeutic proteins to improve their stability in vivo without compromising bioactivity.
Graphical Abstract
Flashback
50 Years Ago ...
- Page: 6802
- First Published: 21 June 2013
Angewandte Chemie International Edition was first published in 1962, the mother journal first in 1888. In this monthly flashback, we feature some of the articles that appeared 50 years ago. This look back can open our eyes, stimulate discussion, or even raise a smile.
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 27/2013
- Pages: 6808-6810
- First Published: 21 June 2013
Author Profile
News
New Members of the National Academy of Sciences Royal Society Wolfson Research Merit Awards
- Pages: 6814-6815
- First Published: 13 June 2013
Highlight
Trifluoromethylthiolation
Formation of CSCF3 Bonds through Direct Trifluoromethylthiolation†
- Pages: 6818-6819
- First Published: 09 May 2013
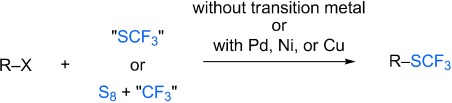
Modern chemistry with an old substituent: The introduction of the SCF3 group into organic substrates is a challenging task because of harsh or specific synthetic methods. However, recent advances in the formation of CSCF3 bonds include the trifluoromethylthiolation with transition-metal-free systems or in the presence of palladium, nickel, or copper catalysts (see scheme).
Review
Non-natural Translation
Reactions Templated by Nucleic Acids: More Ways to Translate Oligonucleotide-Based Instructions into Emerging Function
- Pages: 6820-6843
- First Published: 12 June 2013
Not lost in translation: The programmability of oligonucleotide recognition offers an attractive platform to direct the assembly of reactive partners so that a functional output is achieved. Recent progress in the type of transformations and their applications (such as the translation of oligonucleotide information in functional materials and novel architectures, bioactivity, or fluorescence) are summarized.
Communications
Photochemistry
Fabrication of Complex Three-Dimensional Polymer Brush Nanostructures through Light-Mediated Living Radical Polymerization†
- Pages: 6844-6848
- First Published: 03 June 2013
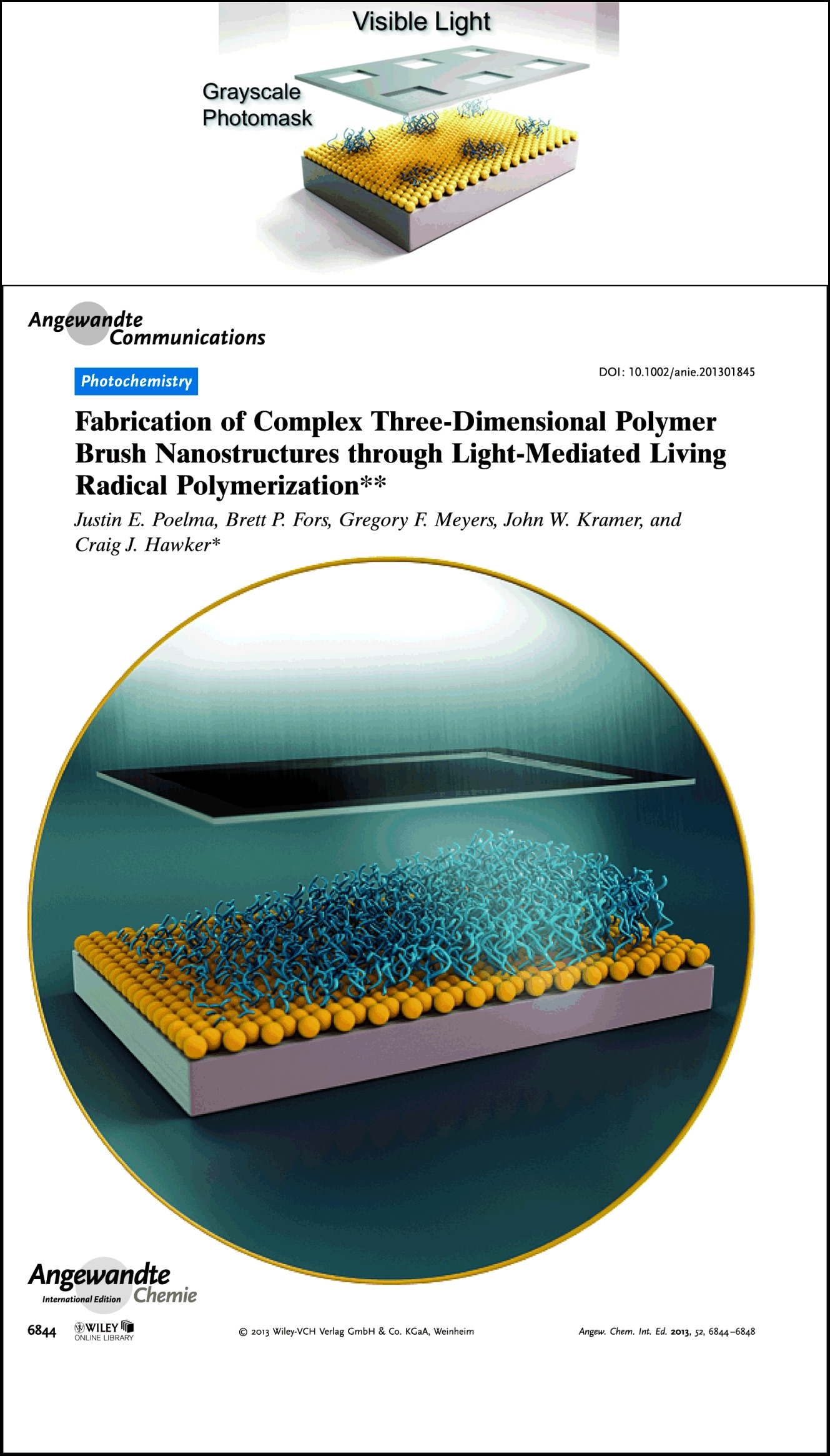
A facile approach to unique 3D, patterned polymer brushes is based on visible-light-mediated controlled radical polymerization. The temporal and spatial control of the polymerization allows the patterning of polymer brushes from a uniform initiating layer using a simple photomask (see picture). Furthermore, gradient polymer brushes, patterned block copolymers, and complex 3D structures can be obtained by modulating light intensity.
Host–Guest Systems
Guest-Induced Unidirectional Dual Rotary and Twisting Motions of a Spiroborate-Based Double-Stranded Helicate Containing a Bisporphyrin Unit†
- Pages: 6849-6853
- First Published: 28 May 2013
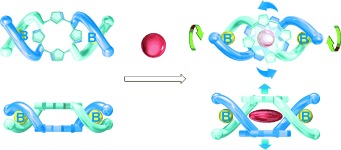
Influential guests: The intercalation of an electron-deficient aromatic guest (shown in red) between the two porphyrin rings of an optically active, porphyrin-linked double-stranded spiroborate helicate triggered rotary motion of the porphyrin rings in one direction in conjunction with a unidirectional twisting motion of the spiroborate helix. This system has potential for the development of chirality-responsive molecular machines.
DNA Nanocapsules
Controlled Release of Encapsulated Cargo from a DNA Icosahedron using a Chemical Trigger†
- Pages: 6854-6857
- First Published: 28 May 2013
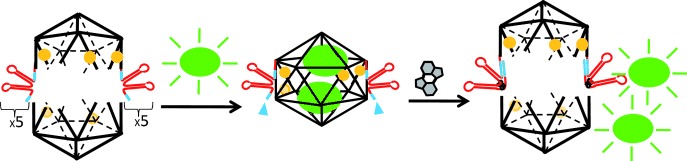
DNA Trojan horse: A DNA icosahedron (black, see scheme) held together with aptamers (red) was used to encapsulate molecular cargo such as fluorescent dextran (green). In the presence of a molecular trigger (gray hexagons), the aptamers fold back leading to opening of the icosahedron and simultaneous release of the encapsulated cargo.
Nanostructures
Synthesis of Enantiopure Carbonaceous Nanotubes with Optical Activity†
- Pages: 6858-6862
- First Published: 28 May 2013
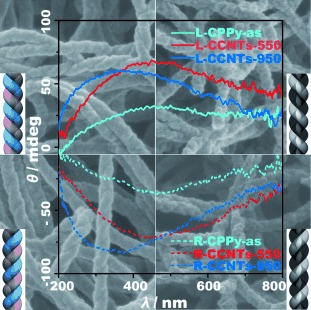
In one step, self-assembled helical polypyrrole nanotubes were carbonized to enantiopure chiral carbonaceous nanotubes with a partially graphitized nanostructure (see picture). The ordered helical arrangement of the carbon nanostructure resulted in enantiomeric materials with distinct optical activity. Moreover, their unique structure endowed them with high reversible storage capacity of lithium ions.
Liquid Crystals
A General Method for the Enantioselective Formation of Helical Nanofilaments†
- Pages: 6863-6866
- First Published: 28 May 2013
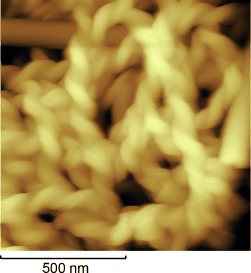
Controlling chirality: A general method to obtain homochiral helical nanofilaments (HNFs) based on a twisted nematic (TN) configuration was developed. By mixing bent-core molecules in the B4 phase with rod-like molecules in the nematic phase, the mixtures show the phase sequence of N–Bx(B4/N). Homochiral HNFs in the Bx phase were obtained from the mixtures when TN cells were cooled. The homochiral HNFs were observed by atomic force microscopy (see picture).
Fuel Cells
Optimized Synthesis of Fe/N/C Cathode Catalysts for PEM Fuel Cells: A Matter of Iron–Ligand Coordination Strength†
- Pages: 6867-6870
- First Published: 29 May 2013
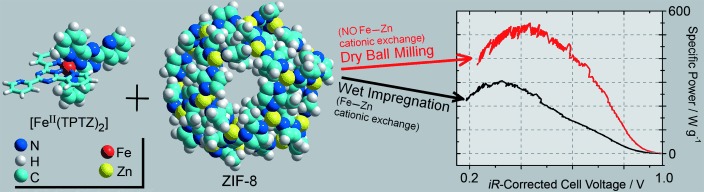
It's all in the detail: Highly active Fe/N/C catalysts for the oxygen-reduction reaction in polymer-electrolyte-membrane (PEM) fuel cells can be obtained from a precursor constructed from the zeolitic imidazolate framework ZIF-8 and an iron–ligand complex if, and only if, the displacement of ZnII ions from ZIF-8 by FeII ions is restricted (see picture; TPTZ=2,4,6-tris(2-pyridyl)-s-triazine).
Femtochemistry
Ultrafast Solvent-Assisted Electronic Level Crossing in 1-Naphthol†
- Pages: 6871-6875
- First Published: 12 June 2013
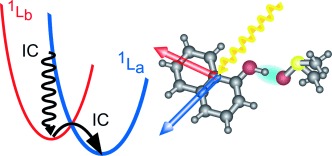
Solvent effects: The nonadiabatic inversion dynamics of the energetic order of the electronic excited states of the photoacid 1-naphthol have been revealed by ultrafast spectroscopy on a femtosecond timescale (see picture; IC=internal conversion). The energetic order of the excited states La and Lb of 1-naphthol is reversed in 60 fs in polar dimethyl sulfoxide solvent.
High-Nitrogen Compounds
Electroactive Explosives: Nitrate Ester-Functionalized 1,2,4,5-Tetrazines†
- Pages: 6876-6879
- First Published: 16 May 2013
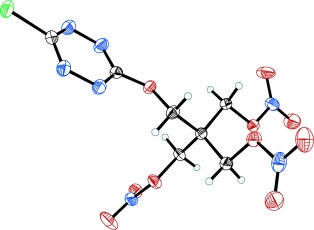
Oxidation state control: The synthesis and characterization of two nitrate ester-functionalized electroactive tetrazine molecules (see example; N blue, Cl green, O red, C black) has been accomplished. The compounds are redox active and have desirable explosive properties. The reversible nature of their reduction could be utilized to control their chemical and physical properties.
Protein Modifications
Mix to Validate: A Facile, Reversible PEGylation for Fast Screening of Potential Therapeutic Proteins In Vivo†
- Pages: 6880-6884
- First Published: 05 June 2013
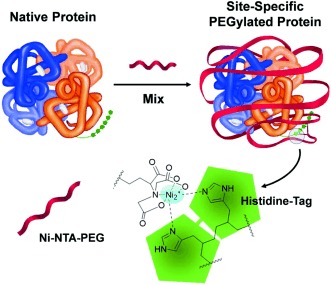
Happy TRAILs to you: PEGylation of proteins through complementary interactions between a His-tag and a Ni2+ complex of nitrilotriacetic acid (NTA, see picture), a well-established practice in protein research, was used to improve the half-life of therapeutic proteins in the blood following systemic administration in vivo. Animal models show that this site-specific modification improves the efficacy of modified TRAIL proteins.
Metal Polycations
A Tetrapositive Metal Ion in the Gas Phase: Thorium(IV) Coordinated by Neutral Tridentate Ligands†
- Pages: 6885-6888
- First Published: 06 May 2013
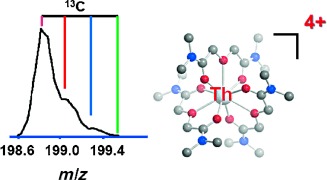
Sheltering thorium ions: A Th4+ ion supported by three neutral tetramethyl-3-oxaglutaramide ligands (L=TMOGA) is produced in the gas phase by electrospray ionization (see graph). The thorium in chiral Th(L)34+ is coordinated by nine oxygen atoms (see picture; O red, N blue, C gray). Quantum chemical studies revealed a decrease in ThO binding energies and bond orders and an increase in bond lengths, as the number of coordinating ligands increases.
Photoresponsive Nanowires
Photoinduced Curling of Organic Molecular Crystal Nanowires†
- Pages: 6889-6893
- First Published: 16 May 2013
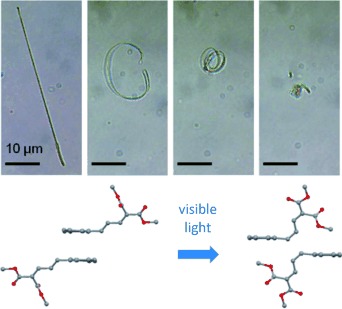
Molecular crystal nanowires composed of an anthracene-9-(1,3-butadiene) derivative exhibit a rapid transition from straight to highly coiled structures when exposed to a pulse of visible light. The curling does not depend on the direction of light illumination and occurs for nanowires composed of either the E or Z isomer. The shape change is driven by an E⇄Z photoisomerization reaction that generates a mixture of isomers within a single nanowire.
Supramolecular Medicine
Supramolecular Iron Porphyrin/Cyclodextrin Dimer Complex that Mimics the Functions of Hemoglobin and Methemoglobin†
- Pages: 6894-6897
- First Published: 27 May 2013
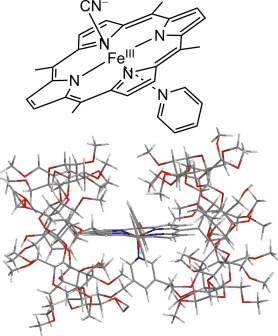
A real life saver: A supramolecule (met-hemoCD3, see scheme) was prepared by including a water-soluble porphinato iron(III) in a capsule of a cyclodextrin dimer (Py3OCD), which was synthesized in only two steps. The structure of met-hemoCD3 was determined by X-ray crystal analysis (see picture). Ferrous hemoCD3 showed functions similar to those of hemoglobin/myoglobin and also functioned as an antidote for cyanide poisoning.
Supramolecular Chemistry
Highly Cooperative Binding of Ion-Pair Dimers and Ion Quartets by a Bis(calix[4]pyrrole) Macrotricyclic Receptor†
- Pages: 6898-6902
- First Published: 27 May 2013
POMcycles
Template-Directed Assembly of Polyoxothiometalate Scaffolds into Nanomolecular Architectures†
- Pages: 6903-6906
- First Published: 28 May 2013
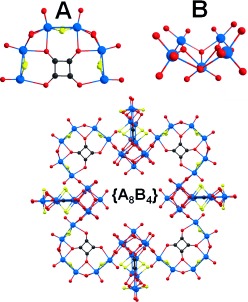
A new family of nanomolecular clusters was obtained by using the squarate anion as a template for the self-assembly of [Mo2O2S2(H2O)6]2+ (A) with the structure-directing anion [Mo5O18]6− (B) whose geometry dictates the formation of the polyoxothiometalate building blocks [(Mo2O2S2)3(OH)4(C4O4)] and [(Mo2O2S2)2(OH)2(C4O4)]. A gradual evolution in both symmetry and structural completeness is observed upon traversing the generations of the POMcycle family.
Molecular Imprinting
Thermo-Responsive Hydrogel Layers Imprinted with RGDS Peptide: A System for Harvesting Cell Sheets†
- Pages: 6907-6911
- First Published: 28 May 2013
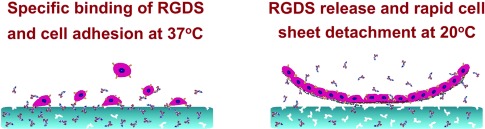
The efficient promotion of cell adhesion on a polymer surface and detachment of sheets of cells is possible with thermo-responsive cell culture substrates that are biofunctionalized through noncovalent molecular imprinting. The key is the thermo-responsive “specific binding” of the cell-adhesive peptide RGDS on the cell sheet harvest system (see picture).
Phosphorylation
Synthesis of Unsymmetric Diphospho-Inositol Polyphosphates†
- Pages: 6912-6916
- First Published: 27 May 2013
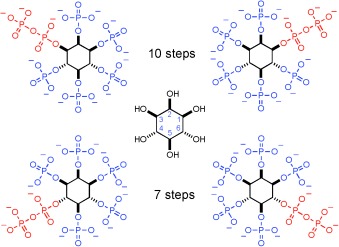
One to rule them all: A novel C2-symmetric phosphoramidite was developed that can be used to prepare all four unsymmetric diphospho inositol pentaphosphates (PP-InsP5). The target structures were synthesized in few steps and high enantiomeric ratios. With the obtained compounds, specificity of Ddp1 from yeast (a PP-InsP5 phosphatase) was studied.
Fluorogenic Probes
BODIPY–Tetrazine Derivatives as Superbright Bioorthogonal Turn-on Probes†
- Pages: 6917-6920
- First Published: 27 May 2013
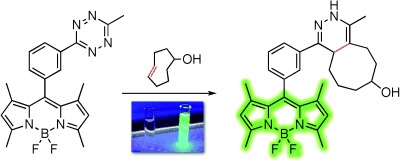
The fastest and the brightest: A new design that intimately connects tetrazine to a BODIPY fluorophore enables exceptionally efficient energy transfer and quenching. Upon reaction of the tetrazine, the brightness of the fluorophore increases more than a thousand-fold, which is a fluorogenic activation up to two orders of magnitude greater than previously described.
Antitumor Agents
The Two Faces of Potent Antitumor Duocarmycin-Based Drugs: A Structural Dissection Reveals Disparate Motifs for DNA versus Aldehyde Dehydrogenase 1 Affinity†
- Pages: 6921-6925
- First Published: 16 May 2013
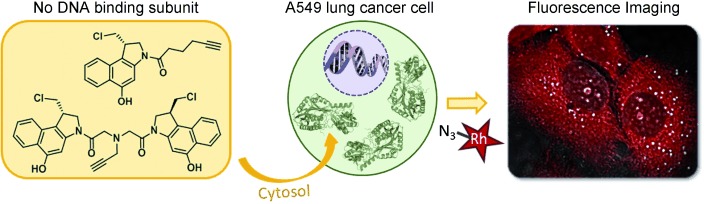
Duocarmycin-derived seco-cyclopropabenzindole (CBI) drugs have been shown to bind DNA and an aldehyde dehydrogenase (ALDH1A1) in lung cancer cells. The removal of the DNA-binding indole moiety results in a CBI compound that does not bind to DNA in whole cells but still exhibits remarkable cytotoxicity. This CBI compound has an increased affinity for ALDH1A1. Rh=rhodamine.
Targeted Delivery
A Reactive Oxygen Species (ROS)-Responsive Polymer for Safe, Efficient, and Targeted Gene Delivery in Cancer Cells†
- Pages: 6926-6929
- First Published: 28 May 2013
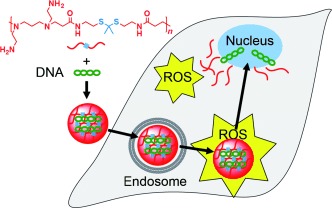
Stimuli-responsive release: The high levels of reactive oxygen species (ROS) in prostate cancer cells can be exploited to trigger cancer-targeted gene delivery. A ROS-responsive thioketal-based cationic polymer was synthesized and functionalization with a cancer-targeting peptide led to selective and enhanced gene transfection in prostate cancer cells (see scheme).
Lithium–Sulfur Batteries
Highly Reversible Lithium/Dissolved Polysulfide Batteries with Carbon Nanotube Electrodes†
- Pages: 6930-6935
- First Published: 29 May 2013
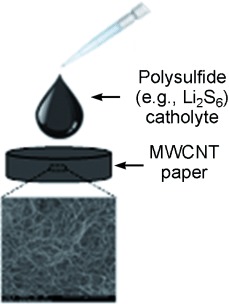
On paper: A lithium/dissolved polysulfide cell is developed utilizing a self-weaving, free-standing multiwalled carbon nanotube (MWCNT) “paper” as a host for the dissolved polysulfide active material and the reaction products. Exceptionally high capacities of 1600 mAh g−1 initially and 1411 mAh g−1 after 50 cycles (based on the mass of sulfur) are obtained at a rate of C/10.
Gold Catalysts
Resonant Photoemission Observations and DFT Study of s–d Hybridization in Catalytically Active Gold Clusters on Ceria Nanorods†
- Pages: 6936-6939
- First Published: 28 May 2013
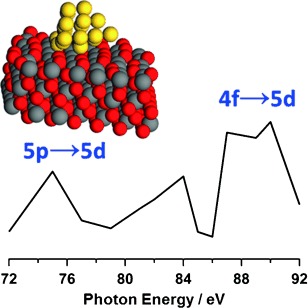
Up shift: 6s–5d hybridization and the up-shift of the 5d band to the Fermi level is demonstrated in catalytically active gold clusters on a cerium oxide nanorod support by using resonant photoemission spectroscopy and density functional theory modeling. These results help to explain the high catalytic activity of this system in CO oxidation.
Expanded Porphyrins
Palladium-Induced Pyrrolic Rearrangement of a Singly to a Doubly N-Confused [26]Hexaphyrin†
- Pages: 6940-6943
- First Published: 23 May 2013
Anticancer Nanofibers
Disruption of the Dynamics of Microtubules and Selective Inhibition of Glioblastoma Cells by Nanofibers of Small Hydrophobic Molecules†
- Pages: 6944-6948
- First Published: 17 May 2013
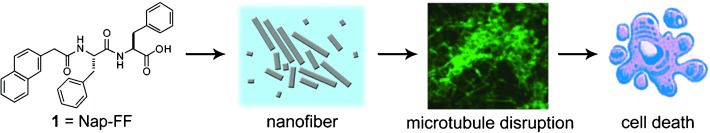
Ganging up against the bad guys: Nanofibers of 1 efficiently inhibited the growth of glioblastoma cells but exhibited little acute toxicity toward a neuronal cell line. The selective cytotoxicity probably stems from the Warburg effect of cancer cells and the existence of microtubule-stabilizing proteins in neurons. Supramolecular nanofibers that can interrupt the self-organization of proteins may have potential as nanomedicines for the treatment of cancer.
Soft Material Chemistry
Tailoring Hydrogel Adhesion to Polydimethylsiloxane Substrates Using Polysaccharide Glue†
- Pages: 6949-6952
- First Published: 31 May 2013
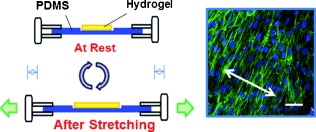
Hydrogel meets silicone: The chemical functionalization of a polydimethylsiloxane (PDMS) surface with polysaccharide “glue” induces a strong, permanent adhesion between the hydrogel and PDMS. This hydrogel-coated silicone substrate was useful for controlling cellular organization under mechanical stretching (see picture) and also in fabricating microfluidic devices filled with the gel.
Cycloaddition
Silver-Catalyzed Isocyanide-Alkyne Cycloaddition: A General and Practical Method to Oligosubstituted Pyrroles†
- Pages: 6953-6957
- First Published: 06 May 2013
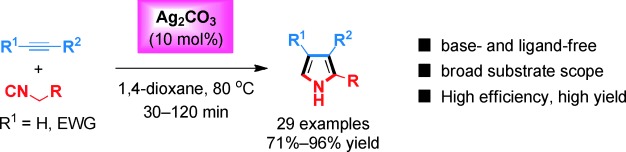
Ag2CO3 is the key: The transition-metal-catalyzed cycloaddition of isocyanides and unactivated terminal alkynes has been realized with Ag2CO3 as a unique and robust catalyst (see scheme). The protocol is highly efficient, allowing a broad range of terminal and internal alkynes to react under base- and ligand-free conditions, generating synthetically useful oligosubstituted pyrroles in high yields.
Synthesis of Pyrroles by Click Reaction: Silver-Catalyzed Cycloaddition of Terminal Alkynes with Isocyanides†
- Pages: 6958-6961
- First Published: 29 April 2013
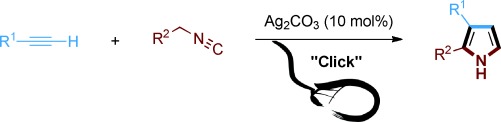
Just click with silver: Pyrroles are prepared by the co-cyclization of terminal alkynes and isocyanides in a silver-catalyzed click reaction. This route represents an extremely simple, efficient, and atom-economic approach to substituted pyrroles in good yields with high selectivity, thus complementing the click method for the rapid formation of multifunctional heterocycles.
Trifluoromethylation
Copper-Catalyzed Trifluoromethylation-Initiated Radical 1,2-Aryl Migration in α,α-Diaryl Allylic Alcohols†
- Pages: 6962-6966
- First Published: 27 May 2013
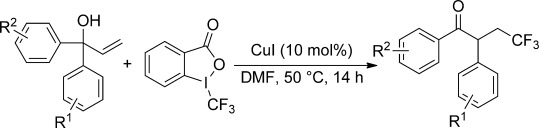
Not only symmetrical, but also unsymmetrical α,α-diaryl allylic alcohols are employed as substrates in the title reaction. A number of arenes and even heteroarenes underwent radical 1,2-aryl migration (“neophyl rearrangement”) to produce α-aryl β-trifluoromethyl ketones. The preferential migration of electron-deficient aryl groups over electron-rich ones in unsymmetrical substrates supports the radical mechanism, which was further confirmed by DFT calculations.
Phosphorus Polymers
Isomerization Polymerization of the Phosphaalkene MesPCPh2: An Alternative Microstructure for Poly(methylenephosphine)s†
- Pages: 6967-6970
- First Published: 05 June 2013
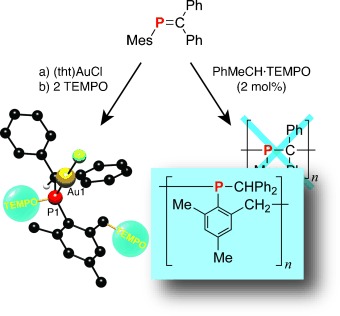
Unique pathway: The radical-initiated addition polymerization of MesP=CPh2 propagates through the ortho-bound CH3 group of the Mes moiety after CH bond activation (see scheme, Mes=2,4,6-trimethylphenyl, tht=tetrahydrothiophene, TEMPO=2,2,6,6-tetramethyl-l-piperidinoxyl). This unique isomerization polymerization mechanism contrasts the previously suggested head-to-tail enchainment typically observed for olefins.
Natural Products
Formal Enantioselective Synthesis of Aplykurodinone-1†
- Pages: 6971-6973
- First Published: 23 May 2013
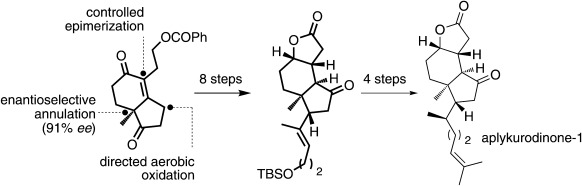
Step economy and simplicity were combined in the asymmetric formal synthesis of aplykurodinone-1 (see scheme; TBS=tert-butyldimethylsilyl). The key features of the strategy involve a one-pot aerobic and directed oxidation/deoxygenation and a late-stage controlled epimerization to form the chiral architecture of the molecule.
Ionic Silicon(II) Compounds
Silicon(II) Coordination Chemistry: N-Heterocyclic Carbene Complexes of Si2+ and SiI+†
- Pages: 6974-6978
- First Published: 27 May 2013
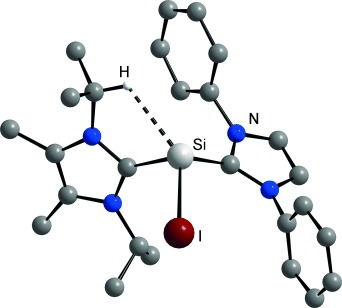
Ligand swap: The exchange of N-heterocyclic carbene (NHC) ligands at SiII centers is shown to provide access to a dicationic NHC complex of silicon(II), and an NHC adduct of the iodosilyliumylidene cation SiI+, [SiI(IiPr2Me2)(IDipp)]+ (see picture). Characterization studies led to the discovery of an unprecedented CH⋅⋅⋅Si anagostic interaction for [SiI(IiPr2Me2)(IDipp)]+.
Peptide Nanoassemblies
Towards Structure Determination of Self-Assembled Peptides Using Dynamic Nuclear Polarization Enhanced Solid-State NMR Spectroscopy†
- Pages: 6979-6982
- First Published: 05 April 2013
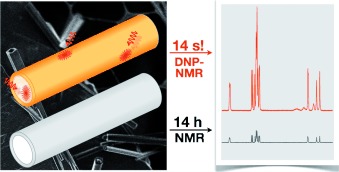
Supra-sensitivity: Dynamic nuclear polarization (DNP) enhanced solid-state NMR spectroscopy was performed on self-assembled peptide nanotubes. This approach yields significant experimental time savings (about five orders of magnitude; see picture) and was used to exemplify the feasibility of supramolecular structural studies of organic nanoassemblies at an atomic scale using DNP-enhanced solid-state NMR spectroscopy.
Synthetic Methods
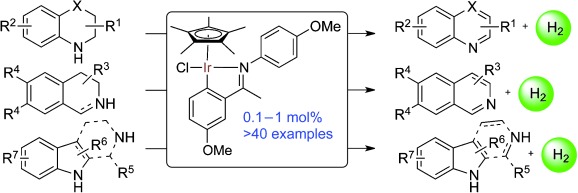
Acceptorless Dehydrogenation of Nitrogen Heterocycles with a Versatile Iridium Catalyst†
- Pages: 6983-6987
- First Published: 17 May 2013
Asymmetric Synthesis
Methylene-Bridged Bis(imidazoline)-Derived 2-Oxopyrimidinium Salts as Catalysts for Asymmetric Michael Reactions†
- Pages: 6988-6991
- First Published: 27 May 2013
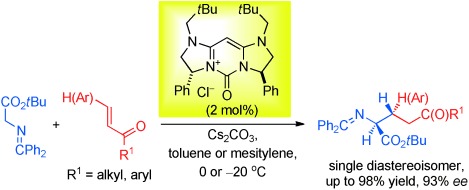
In nothing flat: The title salts, having planar nitrogen centers, were utilized successfully as phase-transfer catalysts for asymmetric Michael reactions of tert-butyl glycinate benzophenone Schiff base with vinyl ketone and chalcone derivatives, thus providing excellent levels of diastereo- and enantiocontrol (see scheme).
Kinetics
Model Studies of the Kinetics of Ester Hydrolysis under Stretching Force†
- Pages: 6992-6995
- First Published: 17 May 2013
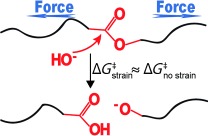
Experiments and computations are reported of how stretching a polymer containing an ester moiety affects the kinetics of its basic hydrolysis (see picture). DFT computations of complete conformational ensembles of three homologous esters suggest that a stretching force stabilizes the tetrahedral intermediate and the second transition state (TS) but has no effect on the relative energy of the first TS.
Supported Catalysts
Continuous Gas-Phase Hydroaminomethylation using Supported Ionic Liquid Phase Catalysts†
- Pages: 6996-6999
- First Published: 16 May 2013
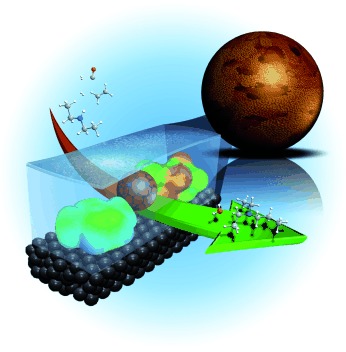
Just SILP-ing through: Hydroaminomethylation of ethylene and diethylamine to diethylpropylamine is demonstrated as a continuous gas-phase reaction (see picture) using a supported ionic liquid phase (SILP) to immobilize the applied homogenous Rh-Xantphos catalyst. Highly selective and long-term stable (18 days) catalyst operation was obtained if the ionic liquid was of low basicity and lipophilicity combined with a porous activated carbon support.
Autonomous Nanopropulsion
Self-Propelled Polymer-Based Multilayer Nanorockets for Transportation and Drug Release†
- Pages: 7000-7003
- First Published: 23 May 2013
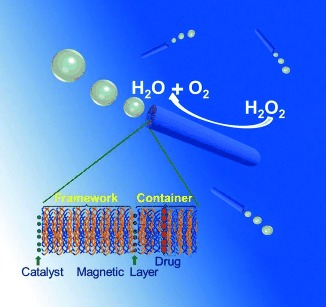
Nanotransporters: Self-assembled polymer multilayer nanorockets based on a template-assisted layer-by-layer technique can self-propel by chemical power, namely hydrogen peroxide degradation. They can perform drug loading, targeted transportation, and triggered drug release by an external physical stimuli in a controlled manner.
Continuous Translation Reaction
Rolling Circle Amplification in a Prokaryotic Translation System Using Small Circular RNA†
- Pages: 7004-7008
- First Published: 28 May 2013
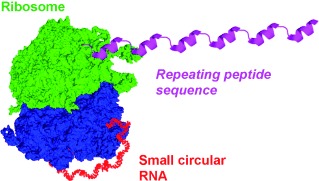
Getting the runaround: Small circular RNA molecules containing an infinite open reading frame were synthesized and tested in an E. coli cell-free translation system. A circular RNA 126 nucleotides in length was found to produce more product than its linear counterpart by two orders of magnitude, because a ribosome can work more effectively towards the elongation on circular RNA than it can on linear RNA in this continuous peptide synthesis.
Homogeneous Catalysis
Triphenylene-Based Tris(N-Heterocyclic Carbene) Ligand: Unexpected Catalytic Benefits†
- Pages: 7009-7013
- First Published: 03 June 2013
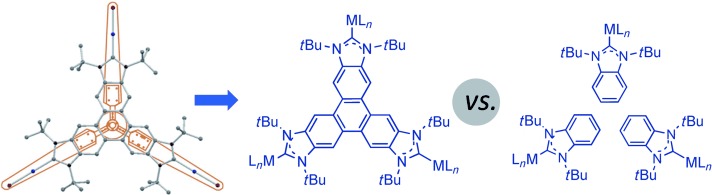
Triple play: A novel triphenylene-based tris(N-heterocyclic carbene) ligand with D3h symmetry and a highly π-delocalized system has been prepared and coordinated to palladium and gold (see figure). The catalytic activities of the new complexes have been compared with those of related benzimidazolylidene and a triptycene-based tris(N-heterocyclic carbene) complexes in three reactions.
Stable Radicals
Stereoselective Rearrangement
Stereoselective Rearrangements with Chiral Hypervalent Iodine Reagents†
- Pages: 7018-7022
- First Published: 07 May 2013
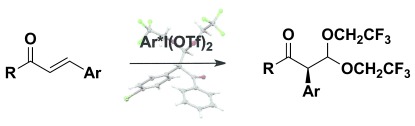
I likes rearrangements: Hypervalent iodine compounds can be used as environmentally friendly, mild, and selective reagents for highly enantioselective rearrangements of alkenes. For the first time, rearrangements to α-arylated ketones can be performed enantioselectively using lactic acid-based iodine(III) reagents.
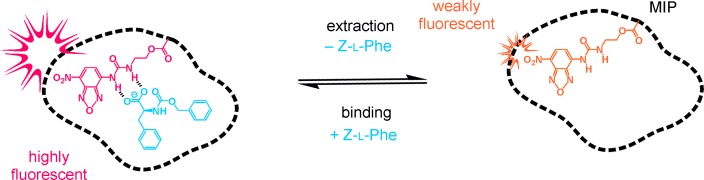
Sensor Particles
Fluorescent Sensory Microparticles that “Light-up” Consisting of a Silica Core and a Molecularly Imprinted Polymer (MIP) Shell†
- Pages: 7023-7027
- First Published: 28 May 2013
Halogen Bonds
Organocatalysis by Neutral Multidentate Halogen-Bond Donors†
- Pages: 7028-7032
- First Published: 06 May 2013
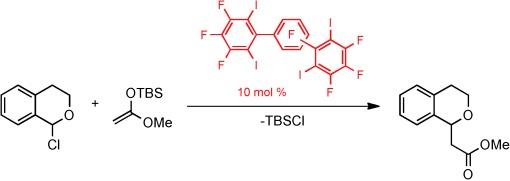
I(n)organocatalysis: Neutral multidentate halogen-bond donors (halogen-based Lewis acids) catalyze the reaction of 1-chloroisochroman with ketene silyl acetals. The organocatalytic activity is linked to the presence (and number as well as orientation) of iodine substituents. As hidden acid catalysis can be ruled out with high probability, this case constitutes strong evidence for halogen-bond based organocatalysis. TBS=tert-butyldimethylsilyl.
Pyrrolysine Biosynthesis
Structure and Reaction Mechanism of Pyrrolysine Synthase (PylD)†
- Pages: 7033-7037
- First Published: 29 May 2013
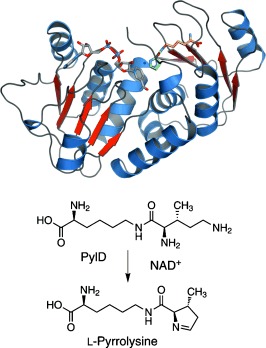
The final step in the biosynthesis of the 22nd genetically encoded amino acid, pyrrolysine, is catalyzed by PylD, a structurally and mechanistically unique dehydrogenase. This catalyzed reaction includes an induced-fit mechanism achieved by major structural rearrangements of the N-terminal helix upon substrate binding. Different steps of the reaction trajectory are visualized by complex structures of PylD with substrate and product.
Boron Ligands
An Electron-Precise, Tetrahedral μ3 Boride Complex†
- Pages: 7038-7041
- First Published: 03 June 2013
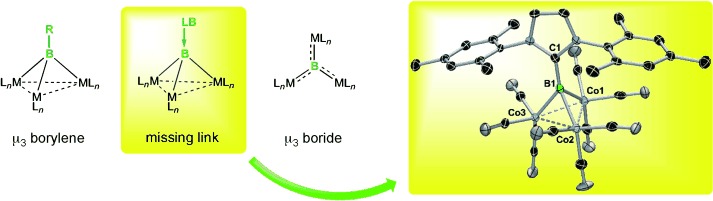
Boron at the top: Reaction of IMes⋅BBr3 (IMes=1,3-bis(2,4,6-trimethylphenyl) imidazol-2-ylidene) with Na[Co(CO)4] afforded the trimetalloboride [(CO)9Co3(μ3-B⋅IMes)] with high selectivity. This species has a tetrahedral geometry with an electron-precise bonding situation, which is uncommon for a μ3 boride complex. It can be considered the missing link between a tetrahedral μ3 borylene (nonclassical) and planar μ3 boride (electron-precise) complexes.






![Highly Cooperative Binding of Ion-Pair Dimers and Ion Quartets by a Bis(calix[4]pyrrole) Macrotricyclic Receptor](/cms/asset/e8d8ccaf-01f2-4086-9a23-6fb82d95ec0a/mcontent.jpg)
![Palladium-Induced Pyrrolic Rearrangement of a Singly to a Doubly N-Confused [26]Hexaphyrin](/cms/asset/3d49df23-8b58-4981-a5b1-21542a3a8e24/mcontent.jpg)