Acceptorless Dehydrogenation of Nitrogen Heterocycles with a Versatile Iridium Catalyst†
Jianjun Wu
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
These authors contributed equally to this work.
Search for more papers by this authorDinesh Talwar
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
These authors contributed equally to this work.
Search for more papers by this authorSteven Johnston
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
Search for more papers by this authorProf. Ming Yan
Institute of Drug Synthesis and Pharmaceutical Process, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006 (China)
Search for more papers by this authorCorresponding Author
Prof. Jianliang Xiao
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)Search for more papers by this authorJianjun Wu
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
These authors contributed equally to this work.
Search for more papers by this authorDinesh Talwar
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
These authors contributed equally to this work.
Search for more papers by this authorSteven Johnston
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
Search for more papers by this authorProf. Ming Yan
Institute of Drug Synthesis and Pharmaceutical Process, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006 (China)
Search for more papers by this authorCorresponding Author
Prof. Jianliang Xiao
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)
Department of Chemistry, University of Liverpool, Liverpool L69 7ZD (UK)Search for more papers by this authorWe thank the EPSRC and the University of Liverpool for a DTA studentship, Dr. Weijun Tang, Yi Zhang, and Dr. Jonathan H. Barnard for assistance and the EPSRC NMSSC at Swansea University for mass analysis.
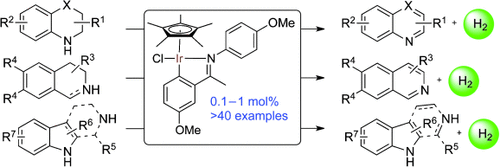
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201300292_sm_miscellaneous_information.pdf685 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. J. Arpe, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 2010.
- 2
- 2aG. E. Dobereiner, R. H. Crabtree, Chem. Rev. 2010, 110, 681;
- 2bJ. Choi, A. H. R. MacArthur, M. Brookhart, A. S. Goldman, Chem. Rev. 2011, 111, 1761.
- 3Examples of heterogeneous CDH:
- 3aH. Adkins, L. G. Lundsted, J. Am. Chem. Soc. 1949, 71, 2964;
- 3bT. Hara, K. Mori, T. Mizugaki, K. Ebitani, K. Kaneda, Tetrahedron Lett. 2003, 44, 6207;
- 3cZ. Wang, I. Tonks, J. Belli, C. M. Jensen, J. Organomet. Chem. 2009, 694, 2854;
- 3dD. Dean, B. Davis, P. G. Jessop, New J. Chem. 2011, 35, 417.
- 4Apart from a few examples of hydroquinolines CDH (Ref. [5]), only three indolines have been repoted to undergo homogeneous CDH:
- 4aY. Tsuji, S. Kotachi, K. T. Huh, Y. Watanabe, J. Org. Chem. 1990, 55, 580;
- 4bS. M. Lu, Y. Q. Wang, X. W. Han, Y. G. Zhou, Chin. J. Catal. 2005, 26, 287.
- 5R. Yamaguchi, C. Ikeda, Y. Takahashi, K. Fujita, J. Am. Chem. Soc. 2009, 131, 8410.
- 6
- 6aL. D. Quin, J. Tyrell, Fundamentals of Heterocyclic Chemistry: Importance in Nature and in the Synthesis of Pharmaceuticals, Wiley, New York, 2010;
- 6bR. H. Crabtree, Energy Environ. Sci. 2008, 1, 134.
- 7
- 7aC. Wang, A. Pettman, J. Basca, J. L. Xiao, Angew. Chem. 2010, 122, 7710; Angew. Chem. Int. Ed. 2010, 49, 7548;
- 7bC. Wang, B. Villa-Marcos, J. L. Xiao, Chem. Commun. 2011, 47, 9773.
- 8However, 1 differs from the Fujita–Yamaguchi catalyst not only structurally but also probably mechanistically. Containing no bifunctional ligand, the hydride generated from 1 can only be protonated intermolecularly. In contrast, [Cp*Ir(2-hydroxypyridine)] operates by ligand-promoted dehydrogenation:
- 8aK. Fujita, N. Tanino, R. Yamaguchi, Org. Lett. 2007, 9, 109;
- 8bH. Li, J. Jiang, G. Lu, F. Huang, Z. X. Wang, Organometallics 2011, 30, 3131.
- 9Formation of H2 was confirmed by GC analysis and quantified with the water displacement method. Please see the Supporting Information for details.
- 10Alcohols are known acceptors for halide anions, see:
- 10aD. K. Smith, Org. Biomol. Chem. 2003, 1, 3874;
- 10bJ. W. Ruan, J. L. Xiao, Acc. Chem. Res. 2011, 44, 614.
- 11In line with dihydrogen formation being turnover-limiting, Ir-H was observed in the 1H NMR spectrum at RT, 5 min after mixing 2 a with 1 d (1 mol %) in TFE in a NMR tube, along with ca. 4 % of 3 a.
- 12TFE can hydrogen bond with and protonate metal hydrides, thus forming M-(H2):
- 12aE. I. Gutsul, N. V. Belkova, M. S. Sverdlov, L. M. Epstein, E. S. Shubina, V. I. Bakhmutov, T. N. Gribanova, R. M. Minyaev, C. Bianchini, M. Peruzzini, F. Zanobini, Chem. Eur. J. 2003, 9, 2219;
- 12bM. Besora, A. Lledós, F. Maseras, Chem. Soc. Rev. 2009, 38, 957.
- 13To further demonstrate that the high activity of this CDH results from the combination of 1 d and TFE, that is, a solvent-assisted CDH, we compared 1 d and the Fujita–Yamaguchi catalyst. Under the conditions of Table 1, the later afforded less than 2 % conversion. In contrast, the conversion was less than 1 % with the former but 77 % with the latter under Fujita’s conditions (2 mol %, p-xylene, reflux, 20 h).
- 14X.-B. Zhang, Z. Xi, Phys. Chem. Chem. Phys. 2011, 13, 3997.
- 15
- 15aG. Cholewinski, K. Dzierzbicka, A. M. Kolodziejczyk, Pharmacol. Rep. 2011, 63, 305;
- 15bM. C. Pirrung, J. H. L. Chau, J. Chen, Chem. Biol. 1995, 2, 621.
- 16R. D. Myers, Experientia 1989, 45, 436.
- 17
- 17aR. H. Manske, Chem. Rev. 1942, 30, 145;
- 17bK. C. Nicolaou, C. J. N. Mathison, T. Montagnon, J. Am. Chem. Soc. 2004, 126, 5192.
- 18J. P. Marino, R. D. Larson, Jr., J. Am. Chem. Soc. 1981, 103, 4642.
- 19L. Kurti, B. Czako, Strategic Applications of Named Reactions in Organic Synthesis, Elsevier Academic Press, London, 2005.
- 20D. Liu, G. Zhao, L. Xiang, Eur. J. Org. Chem. 2010, 3975.
- 21M. Ahmed Ibrahim, Heterocycles 2011, 83, 2689.
- 22J. K. Liu, W. T. Couldwell, Neurocrit. Care 2005, 2, 124.
- 23J. Ishida, H. K. Wang, K. F. Bastow, C. Q. Hu, K. H. Lee, Bioorg. Med. Chem. Lett. 1999, 9, 3319.
- 24
- 24aC. D. Gilmore, K. M. Allan, B. M. Stoltz, J. Am. Chem. Soc. 2008, 130, 1558;
- 24bA. Metzger, M. A. Schade, P. Knochel, Org. Lett. 2008, 10, 1107;
- 24cD. J. Schipper, L. C. Campeau, K. Fagnou, Tetrahedron 2009, 65, 3155.
- 25R. S. Kusurkar, S. K. Goswami, Tetrahedron 2004, 60, 5315.
- 26This suggests again that dehydogenation, without releasing H2, is a fast process. Also see Ref. [11].
- 27When 1 d was heated in TFE at 60 °C, an Ir-H hydride resonance was observed at δ=−10.10 ppm along with trifluoroacetaldehyde in the 1H NMR spectrum (see the Supporting Information).