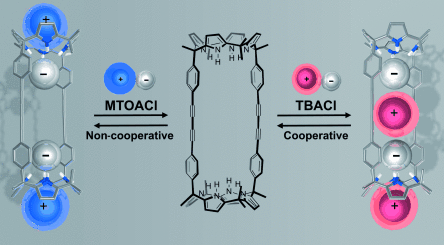
Highly Cooperative Binding of Ion-Pair Dimers and Ion Quartets by a Bis(calix[4]pyrrole) Macrotricyclic Receptor†
Virginia Valderrey
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain) http://www.iciq.es/portal/325/default.aspx
Search for more papers by this authorEduardo C. Escudero-Adán
X-Ray Diffraction Unit, Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Prof. Pablo Ballester
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain) http://www.iciq.es/portal/325/default.aspx
Catalan Institution for Research and Advanced Studies (ICREA), Passeig Lluís Companys, 23, 08010 Barcelona (Spain)
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain) http://www.iciq.es/portal/325/default.aspxSearch for more papers by this authorVirginia Valderrey
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain) http://www.iciq.es/portal/325/default.aspx
Search for more papers by this authorEduardo C. Escudero-Adán
X-Ray Diffraction Unit, Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain)
Search for more papers by this authorCorresponding Author
Prof. Pablo Ballester
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain) http://www.iciq.es/portal/325/default.aspx
Catalan Institution for Research and Advanced Studies (ICREA), Passeig Lluís Companys, 23, 08010 Barcelona (Spain)
Institute of Chemical Research of Catalonia (ICIQ), Avgda. Països Catalans 16, 43007 Tarragona (Spain) http://www.iciq.es/portal/325/default.aspxSearch for more papers by this authorWe thank the Spanish Ministerio de Economía y Competitividad (CTQ2011-23014), the Generalitat de Catalunya (2009SGR00686), and the ICIQ Foundation for financial support.
Graphical Abstract
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302524_sm_miscellaneous_information.pdf4.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1S. K. Kim, J. L. Sessler, Chem. Soc. Rev. 2010, 39, 3784–3809.
- 2A. J. McConnell, P. D. Beer, Angew. Chem. 2012, 124, 5138–5148;
10.1002/ange.201107244 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 5052–5061.
- 3M. J. Deetz, M. Shang, B. D. Smith, J. Am. Chem. Soc. 2000, 122, 6201–6207.
- 4For recent examples of heterotopic ion-pair receptors exhibiting different biding geometries, see:
- 4aS. K. Kim, J. L. Sessler, D. E. Gross, C. H. Lee, J. S. Kim, V. M. Lynch, L. H. Delmau, B. P. Hay, J. Am. Chem. Soc. 2010, 132, 5827–5836;
- 4bM. Ciardi, F. Tancini, G. Gil-Ramírez, E. C. Escudero Adán, C. Massera, E. Dalcanale, P. Ballester, J. Am. Chem. Soc. 2012, 134, 13121–13132.
- 5S. K. Kim, G. I. Vargas-Zuniga, B. P. Hay, N. J. Young, L. H. Delmau, C. Masselin, C. H. Lee, J. S. Kim, V. M. Lynch, B. A. Moyer, J. L. Sessler, J. Am. Chem. Soc. 2012, 134, 1782–1792.
- 6B. Soberats, L. Martinez, E. Sanna, A. Sampedro, C. Rotger, A. Costa, Chem. Eur. J. 2012, 18, 7533–7542.
- 7P. Mateus, R. Delgado, F. Lloret, J. Cano, P. Brandao, V. Felix, Chem. Eur. J. 2011, 17, 11193–11203.
- 8J. M. Lehn, Pure Appl. Chem. 1980, 52, 2441–2459.
- 9J. Eckelmann, V. Saggiomo, F. D. Sonnichsen, U. Luning, New J. Chem. 2010, 34, 1247–1250.
- 10S. Moerkerke, M. Menand, I. Jabin, Chem. Eur. J. 2010, 16, 11712–11719.
- 11C. A. Hunter, C. M. R. Low, C. Rotger, J. G. Vinter, C. Zonta, Chem. Commun. 2003, 834–835.
- 12P. Goralski, M. Chabanel, Inorg. Chem. 1987, 26, 2169–2171.
- 13A. Bhattacharjee, M. N. Roy, Phys. Chem. Chem. Phys. 2010, 12, 14534–14542.
- 14Y. Marcus, G. Hefter, Chem. Rev. 2006, 106, 4585–4621.
- 15D. E. Gross, F. P. Schmidtchen, W. Antonius, P. A. Gale, V. M. Lynch, J. L. Sessler, Chem. Eur. J. 2008, 14, 7822–7827.
- 16R. Custelcean, L. H. Delmau, B. A. Moyer, J. L. Sessler, W. S. Cho, D. Gross, G. W. Bates, S. J. Brooks, M. E. Light, P. A. Gale, Angew. Chem. 2005, 117, 2593–2598;
10.1002/ange.200462945 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 2537–2542.
- 17V. Valderrey, E. C. Escudero-Adan, P. Ballester, J. Am. Chem. Soc. 2012, 134, 10733–10736.
- 18As indicated in the inset of Figure 3, the value for the stability constant of 22⊂1 complexes can be statistically estimated as αKref2. Using this relationship, stability constants with different dimensions can be compared and the high magnitude of the cooperativity factor be inferred.
- 19A binding constant value of 4.3×102 L mol−1 was reported for the 1:1 complex of TBACl with octamethylcalix[4]pyrrole in CD2Cl2 solution: see Ref. [15].
- 20The direct complexation of a negatively charged ion triplet followed by TBA binding constitutes a plausible alternative mechanism, which does not involve cooperativity. However, the amount of ion triplet in solution should be very low at millimolar concentrations.
- 21The use of binding models considering two set of sites or sequential binding sites afforded multiple mathematical solutions providing sensible fits due to the optimization of at least four fitting variables (K1:1, K2:.1, ΔH1:1, ΔH2:1).
- 22The conformational flexibility of 3 compared to 1 could cause an overestimation in the reported cooperativity factors.
- 23Based on competitive experiments (Supporting Information, Figures S19 and S20), the thermodynamic stability of the herodimer 2 a2 b⊂1 lies between that of the two homodimers 2 a2⊂1 and 2 b2⊂1.
- 24CCDC 930892 (2 a2⊂1), 930893 (2 ab⊂1), and 930894 (2 b2⊂1) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.