Silicon(II) Coordination Chemistry: N-Heterocyclic Carbene Complexes of Si2+ and SiI+†
Corresponding Author
Prof. Dr. Alexander C. Filippou
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)Search for more papers by this authorDipl.-Chem. Yury N. Lebedev
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorDr. Oleg Chernov
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorDipl.-Chem. Martin Straßmann
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorDr. Gregor Schnakenburg
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Alexander C. Filippou
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)Search for more papers by this authorDipl.-Chem. Yury N. Lebedev
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorDr. Oleg Chernov
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorDipl.-Chem. Martin Straßmann
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorDr. Gregor Schnakenburg
Institut für Anorganische Chemie, Universität Bonn, Gerhard-Domagk-Strasse 1, 53121 Bonn (Germany)
Search for more papers by this authorWe thank the Deutsche Forschungsgemeinschaft (SFB 813) for generous financial support of this work. We also thank C. Schmidt, K. Prochnicki, and H. Spitz for recording the solution NMR spectra, Dr. S. Schwieger for collection of the data for one X-ray crystal structure, Dr. W. Hoffbauer for recording the 29Si{1H} MAS-NMR spectrum of 2-I and A. Martens for the elemental analyses.
Graphical Abstract
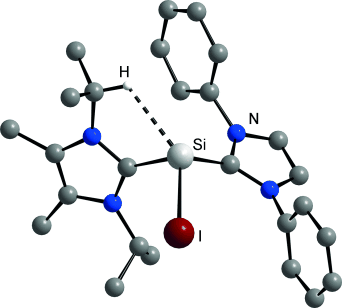
Ligand swap: The exchange of N-heterocyclic carbene (NHC) ligands at SiII centers is shown to provide access to a dicationic NHC complex of silicon(II), and an NHC adduct of the iodosilyliumylidene cation SiI+, [SiI(IiPr2Me2)(IDipp)]+ (see picture). Characterization studies led to the discovery of an unprecedented CH⋅⋅⋅Si anagostic interaction for [SiI(IiPr2Me2)(IDipp)]+.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201301363_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aM. H. Sayyad, E. T. Kennedy, L. Kiernan, J.-P. Mosnier, J. T. Costello, J. Phys. B 1995, 28, 1715;
- 1bR. A. Komara, M. A. Gearba, C. W. Fehrenbach, S. R. Lundeen, J. Phys. B 2005, 38, S 87.
- 2L. Wang, F. Chen, X.-L. Wang, L.-L. Wang, K.-M. Wang, L. Gao, H.-J. Ma, R. Nie, Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 251, 104.
- 3
- 3aP. A. Rupar, V. N. Staroverov, P. J. Ragogna, K. M. Baines, J. Am. Chem. Soc. 2007, 129, 15138;
- 3bP. A. Rupar, V. N. Staroverov, K. M. Baines, Science 2008, 322, 1360;
- 3cM. J. Ward, P. A. Rupar, M. W. Murphy, Y.-M. Yiu, K. M. Baines, T. K. Sham, Chem. Commun. 2010, 46, 7016.
- 4
- 4aL. Pauling, The Nature of the Chemical Bond, 3rd ed., Cornell University Press, Ithaca, New York, 1960;
- 4bA. L. Allred, J. Inorg. Nucl. Chem. 1961, 17, 215;
- 4cD. Bergmann, J. Hinze, Angew. Chem. 1996, 108, 162;
10.1002/ange.19961080205 Google ScholarAngew. Chem. Int. Ed. Engl. 1996, 35, 150.
- 5
- 5aP. Jutzi, A. Mix, B. Rummel, W. W. Schoeller, B. Neumann, H.-G. Stammler, Science 2004, 305, 849;
- 5bP. Jutzi, A. Mix, B. Neumann, B. Rummel, W. W. Schoeller, H.-G. Stammler, A. B. Rozhenko, J. Am. Chem. Soc. 2009, 131, 12137;
- 5cP. Jutzi, K. Leszczynska, B. Neumann, W. W. Schoeller, H.-G. Stammler, Angew. Chem. 2009, 121, 2634;
10.1002/ange.200805749 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 2596.
- 6
- 6aM. Driess, S. Yao, M. Brym, C. van Wüllen, Angew. Chem. 2006, 118, 6882; Angew. Chem. Int. Ed. 2006, 45, 6730;
- 6bA. Meltzer, C. Präsang, M. Driess, J. Am. Chem. Soc. 2009, 131, 7232;
- 6cA. Meltzer, C. Präsang, C. Milsmann, M. Driess, Angew. Chem. 2009, 121, 3216;
10.1002/ange.200900290 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 3170;
- 6dS. Yao, Y. Xiong, M. Driess, Organometallics 2011, 30, 1748, and references therein.
- 7Y. Xiong, S. Yao, S. Inoue, E. Irran, M. Driess, Angew. Chem. 2012, 124, 10221; Angew. Chem. Int. Ed. 2012, 51, 10074.
- 8Y. Wang, Y. Xie, P. Wei, R. B. King, H. F. Schaefer III, P. v. R. Schleyer, G. H. Robinson, Science 2008, 321, 1069.
- 9
- 9aR. S. Ghadwal, H. W. Roesky, S. Merkel, J. Henn, D. Stalke, Angew. Chem. 2009, 121, 5793; Angew. Chem. Int. Ed. 2009, 48, 5683;
- 9bA. C. Filippou, O. Chernov, G. Schnakenburg, Angew. Chem. 2009, 121, 5797;
10.1002/ange.200902431 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 5687;
- 9cA. C. Filippou, O. Chernov, G. Schnakenburg, Chem. Eur. J. 2011, 17, 13574.
- 10
- 10aJ. Li, S. Merkel, J. Henn, K. Meindl, A. Döring, H. W. Roesky, R. S. Ghadwal, D. Stalke, Inorg. Chem. 2010, 49, 775;
- 10bR. S. Ghadwal, S. S. Sen, H. W. Roesky, M. Granitzka, D. Kratzert, S. Merkel, D. Stalke, Angew. Chem. 2010, 122, 4044; Angew. Chem. Int. Ed. 2010, 49, 3952;
- 10cR. S. Ghadwal, H. W. Roesky, M. Granitzka, D. Stalke, J. Am. Chem. Soc. 2010, 132, 10018;
- 10dR. S. Ghadwal, H. W. Roesky, K. Pröpper, B. Dittrich, S. Klein, G. Frenking, Angew. Chem. 2011, 123, 5486; Angew. Chem. Int. Ed. 2011, 50, 5374;
- 10eS. M. I. Al-Rafia, A. C. Malcolm, R. McDonald, M. J. Ferguson, E. Rivard, Chem. Commun. 2012, 48, 1308;
- 10fO. Chernov, Dissertation, Novel Molecular SiII Precursors for Synthesis of the First Compounds with Metal-Silicon Triple Bonds, Universität Bonn, 2012.
- 11
- 11aA. C. Filippou, O. Chernov, G. Schnakenburg, Chem. Eur. J. 2010, 16, 2866;
- 11bH. Cui, C. Cui, Dalton Trans. 2011, 40, 11937.
- 12
- 12aA. C. Filippou, O. Chernov, K. W. Stumpf, G. Schnakenburg, Angew. Chem. 2010, 122, 3368;
10.1002/ange.201000837 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3296;
- 12bA. C. Filippou, O. Chernov, G. Schnakenburg, Angew. Chem. 2011, 123, 1154; Angew. Chem. Int. Ed. 2011, 50, 1122.
- 13The Supporting Information contains the syntheses, analytical, and spectroscopic data of compounds 1–4, as well as selected NMR spectra for compounds 1–4 and crystallographic data for 1⋅3 (CHCl3), 2-I, 3⋅2 (CD2Cl2) and 4⋅C6H5F. CCDC 924156 (1⋅3 (CHCl3)), 924157 (2-I), 924158 (3⋅2 (CD2Cl2)) and 924159 (4⋅C6H5F) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 14A. Bondi, J. Phys. Chem. 1964, 68, 441.
- 15The unweighted mean (xu) value of the SiI bond lengths of 1⋅3 (CHCl3) and the SiC bonds of 3⋅2 CD2Cl2 is given. The standard deviation (σ) of xu (value given in parentheses) was calculated using the equation σ2=Σ(xi−xu)2/n2−n, where xi is the individual value and n=3.
- 16M. Kolonits, M. Hargittai, Struct. Chem. 1998, 9, 349.
- 17The mean value of the length of a SiC(sp2) single bond was derived from a CSD survey of tetravalent, four-coordinate silicon compounds.
- 18Alternatively, [SiI3(Idipp)]+ can be regarded as a donor-acceptor complex of Idipp with the triiodosilylium ion SiI3+. However, the following findings are less supportive of this view, which implies the presence of a SiC dative bond in [SiI3(Idipp)]+, and instead suggest the presence of a covalent SiC bond (see the Supporting Information): The NBO charges of the SiI3 (q=+0.53e) and Idipp fragment (q=+0.47e) have similar values; also, the ZPVE corrected energy required for the SiC bond homolysis to give the fragments SiI3 and Idipp+ (BDEhom.=315.4 kJ mol−1) is similar to that required for the SiC bond heterolysis to give the fragments SiI3+ and Idipp (BDEhet.=330.9 kJ mol−1); furthermore, the SiC bond dissociation energy of [SiI3(Idipp)]+ compares well with those of typical SiC covalent bonds (BDE ca. 370 kJ mol−1)[29] and is considerably higher than those of SiC dative bonds as found in 2-X (BDE ca. 120 kJ mol−1). For studies of donor–acceptor (dative) bonds, see:
- 18aA. Haaland, Angew. Chem. 1989, 101, 1017; Angew. Chem. Int. Ed. Engl. 1989, 28, 992;
- 18bV. Jonas, G. Frenking, M. T. Reetz, J. Am. Chem. Soc. 1994, 116, 8741;
- 18cH. Jiao, P. v. R. Schleyer, J. Am. Chem. Soc. 1994, 116, 7429.
- 19The structure of the Idipp adducts of SiX4 (X=Cl, Br, I) depends on both the X group and the solvent. Thus, chlorinated hydrocarbons such as CH2Cl2 and CHCl3 favor, in the case of X=Br or I, the ionic form [SiX3(Idipp)]X, whereas [SiBr4(Idipp)] is composed of neutral trigonal bipyramidal molecules in toluene or benzene solutions. In comparison, [SiCl4(Idipp)] is nonionic in CH2Cl2 or benzene solutions:
- 19aRefs. [8] and [9b];
- 19bR. S. Ghadwal, S. S. Sen, H. W. Roesky, G. Tavcar, S. Merkel, D. Stalke, Organometallics 2009, 28, 6374.
- 20G. Engelhardt, R. Radeglia, H. Jancke, E. Lippmaa, M. Mägi, Org. Magn. Res. 1973, 5, 561.
- 21E. A. Williams, Annu. Rep. NMR Spectrosc. 1983, 15, 235.
- 22Three independent molecules with very similar geometric parameters were found in the unit cell of 2-I. The unweighted mean (xu) values of the individual bonding parameters of the three crystallographically distinct molecules of 2-I were used in the discussion of the structure of 2-I. The standard deviations (σ) of xu (values given in parentheses) were calculated using the equation σ2=Σ(xi−xu)2/n2−n, where xi is the respective individual value and n is the total number of individual values.
- 23A. J. Arduengo III, H. V. R. Dias, J. C. Calabrese, F. Davidson, Inorg. Chem. 1993, 32, 1541.
- 24A degree of pyramidalization of 100 % corresponds to a sum of angles of 270°, and a degree of pyramidalization of 0 % indicates a planar coordination of the central atom.
- 25H. Bent, Chem. Rev. 1961, 61, 275.
- 26N. Weidemann, G. Schnakenburg, A. C. Filippou, Z. Anorg. Allg. Chem. 2009, 635, 253.
- 27Details of all quantum chemical studies, comparisons of selected calculated and experimental bond parameters of 1+, its dissociation products, 2-X (X=Cl, Br, I) and 4+, and the cartesian atomic coordinates of all calculated structures are given in the Supporting Information.
- 28R. F. W. Bader, Chem. Rev. 1991, 91, 893.
- 29R. Walsh, Acc. Chem. Res. 1981, 14, 246.
- 30
- 30aH. Ottosson, Chem. Eur. J. 2003, 9, 4144, and references therein;
- 30bH. Ottosson, A. M. Eklöf, Coord. Chem. Rev. 2008, 252, 1287.
- 31The reversed SiC polarization of 2-X is evidenced by the NBO charges of the SiX2 fragments: 2-Cl, q(SiCl2)=−0.29e; 2-Br, q(SiBr2)=−0.28e; 2-I, q(SiI2)=−0.27e.
- 32S. Bailleux, M. Bogey, J. Demaison, H. Bürger, M. Senzlober, J. Breidung, W. Thiel, R. Fajgar, J. Pola, J. Chem. Phys. 1997, 106, 10016.
- 33W. M. Boesveld, B. Gehrhus, P. B. Hitchcock, M. F. Lappert, P. v. R. Schleyer, Chem. Commun. 1999, 755.
- 34The ΔEST of the SiX2 and Idipp fragments were calculated in the frozen geometries adopted by the fragments in 2-X, as well as in the minimum (relaxed) geometries. ΔEST (in the frozen geometries): SiCl2 246.9 kJ mol−1, Idipp 381.0 kJ mol−1; SiBr2 222.4 kJ mol−1, Idipp 379.7 kJ mol−1; SiI2 186.5 kJ mol−1, Idipp 378.4 kJ mol−1; ΔEST (in the minimum geometries): SiCl2 222.1 kJ mol−1; SiBr2 199.4 kJ mol−1; SiI2 165.7 kJ mol−1; Idipp 358.3 kJ mol−1 (see the Supporting Information).
- 35The observation of two signals in a 1:2 ratio in the solid-state 29Si{1H} MAS-NMR spectrum of 2 suggests that two of three independent molecules have the same 29Si shielding tensor.
- 36U. Niemann, Z. Naturforsch. B 1975, 30, 202.
- 37An exchange of the N-heterocyclic carbenes to give the neutral intermediate [SiI2(IMe4)] is also conceivable in the first step, but is less probable given the observation that reaction of 2-I with the more bulky imidazol-2-ylidene IiPr2Me2 stops at the SiII salt [SiI(IiPr2Me2)(Idipp)]I (4).
- 38N. Kuhn, T. Kratz, Synthesis 1993, 561.
- 39
- 39aE. Liepinš, I. Birgele, P. Tomsons, E. Lukevics, Magn. Reson. Chem. 1985, 23, 485;
- 39bJ. Ambati, S. E. Rankin, J. Phys. Chem. A 2010, 114, 12613.
- 40For a recent review on through-space nuclear spin-spin couplings, see: J.-C. Hierso, D. Armspach, D. Matt, C. R. Chim. 2009, 12, 1002.
- 41
- 41aY. Zhang, J. C. Lewis, R. G. Bergman, J. A. Ellman, E. Oldfield, Organometallics 2006, 25, 3515;
- 41bM. Brookhart, M. L. H. Green, G. Parkin, Proc. Natl. Acad. Sci. USA 2007, 104, 6908.