Triphenylene-Based Tris(N-Heterocyclic Carbene) Ligand: Unexpected Catalytic Benefits†
Sergio Gonell
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)
Search for more papers by this authorDr. Macarena Poyatos
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)
Search for more papers by this authorCorresponding Author
Prof. Eduardo Peris
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)Search for more papers by this authorSergio Gonell
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)
Search for more papers by this authorDr. Macarena Poyatos
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)
Search for more papers by this authorCorresponding Author
Prof. Eduardo Peris
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)
Dpto. de Química Inorgánica y Orgánica, Universitat Jaume I, Avda. Sos Baynat, 12071 Castellón (Spain)Search for more papers by this authorWe gratefully acknowlegde financial support from the MEC of Spain (CTQ2011-24055/BQU) and Bancaixa (P1.1B2010-02 and P1.1B2011-22). We would also like to thank the Ramón y Cajal program (M.P.). S.G. thanks the Ministerio de Ciencia e Innovación for a fellowship. The authors are grateful to the Serveis Centrals d’Instrumentació Científica (SCIC) of the Universitat Jaume I for providing us with spectroscopic and X-Ray facilities. We also thank Bob Crabtree for thoughtful discussions.
Graphical Abstract
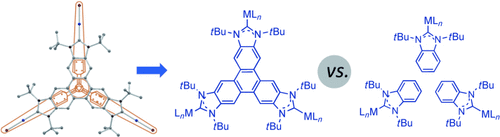
Triple play: A novel triphenylene-based tris(N-heterocyclic carbene) ligand with D3h symmetry and a highly π-delocalized system has been prepared and coordinated to palladium and gold (see figure). The catalytic activities of the new complexes have been compared with those of related benzimidazolylidene and a triptycene-based tris(N-heterocyclic carbene) complexes in three reactions.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201302686_sm_miscellaneous_information.pdf678.3 KB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1R. H. Crabtree, New J. Chem. 2011, 35, 18–23.
- 2
- 2aM. Poyatos, J. A. Mata, E. Peris, Chem. Rev. 2009, 109, 3677–3707;
- 2bF. E. Hahn, M. C. Jahnke, Angew. Chem. 2008, 120, 3166–3216; Angew. Chem. Int. Ed. 2008, 47, 3122–3172.
- 3
- 3aA. Zanardi, J. A. Mata, E. Peris, Chem. Eur. J. 2010, 16, 13109–13115;
- 3bA. Zanardi, J. A. Mata, E. Peris, Organometallics 2009, 28, 1480–1483;
- 3cA. Zanardi, J. A. Mata, E. Peris, Organometallics 2009, 28, 4335–4339;
- 3dA. Zanardi, J. A. Mata, E. Peris, J. Am. Chem. Soc. 2009, 131, 14531–14537;
- 3eA. Zanardi, R. Corberan, J. A. Mata, E. Peris, Organometallics 2008, 27, 3570–3576;
- 3fE. Mas-Marzá, J. A. Mata, E. Peris, Angew. Chem. 2007, 119, 3803–3805;
10.1002/ange.200700535 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 3729–3731.
- 4
- 4aB. M. Neilson, A. G. Tennyson, C. W. Bielawski, J. Phys. Org. Chem. 2012, 25, 531–543;
- 4bB. C. Norris, C. W. Bielawski, Macromolecules 2010, 43, 3591–3593;
- 4cK. A. Williams, A. J. Boydston, C. W. Bielawski, Chem. Soc. Rev. 2007, 36, 729–744;
- 4dA. B. Powell, C. W. Bielawski, A. H. Cowley, Comments Inorg. Chem. 2010, 31, 75–82;
- 4eO. Schuster, L. Mercs, M. Albrecht, Chimia 2010, 64, 184–187.
- 5
- 5aO. Guerret, S. Sole, H. Gornitzka, M. Teichert, G. Trinquier, G. Bertrand, J. Am. Chem. Soc. 1997, 119, 6668–6669;
- 5bA. Zanardi, J. A. Mata, E. Peris, Chem. Eur. J. 2010, 16, 10502–10506;
- 5cA. J. Boydston, C. W. Bielawski, Dalton Trans. 2006, 4073–4077;
- 5dA. J. Boydston, K. A. Williams, C. W. Bielawski, J. Am. Chem. Soc. 2005, 127, 12496–12497;
- 5eD. M. Khramov, A. J. Boydston, C. W. Bielawski, Angew. Chem. 2006, 118, 6332–6335;
10.1002/ange.200601583 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 6186–6189;
- 5fM. D. Sanderson, J. W. Kamplain, C. W. Bielawski, J. Am. Chem. Soc. 2006, 128, 16514–16515;
- 5gA. G. Tennyson, R. J. Ono, T. W. Hudnall, D. M. Khramov, J. A. V. Er, J. W. Kamplain, V. M. Lynch, J. L. Sessler, C. W. Bielawski, Chem. Eur. J. 2010, 16, 304–315;
- 5hA. G. Tennyson, E. L. Rosen, M. S. Collins, V. M. Lynch, C. W. Bielawski, Inorg. Chem. 2009, 48, 6924–6933;
- 5iA. Prades, M. Poyatos, J. A. Mata, E. Peris, Angew. Chem. 2011, 123, 7808–7811;
10.1002/ange.201101806 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 7666–7669;
- 5jA. Prades, E. Peris, M. Alcarazo, Organometallics 2012, 31, 4623–4626.
- 6K. A. Williams, C. W. Bielawski, Chem. Commun. 2010, 46, 5166–5168.
- 7
- 7aS. Carboni, C. Gennari, L. Pignataro, U. Piarulli, Dalton Trans. 2011, 40, 4355–4373;
- 7bL. G. Ma, P. Hao, Q. H. Zhang, D. M. Du, H. X. Xu, Tetrahedron: Asymmetry 2007, 18, 878–884;
- 7cP. Brandt, P. Roth, P. G. Andersson, J. Org. Chem. 2004, 69, 4885–4890;
- 7dR. W. Quan, Z. Li, E. N. Jacobsen, J. Am. Chem. Soc. 1996, 118, 8156–8157;
- 7eG. B. Jones, B. J. Chapman, Synthesis 1995, 475–497;
- 7fH. C. Kolb, P. G. Andersson, K. B. Sharpless, J. Am. Chem. Soc. 1994, 116, 1278–1291;
- 7gL. A. Castonguay, A. K. Rappe, C. J. Casewit, J. Am. Chem. Soc. 1991, 113, 7177–7183.
- 8
- 8aC. M. Crudden, D. P. Allen, Coord. Chem. Rev. 2004, 248, 2247–2273;
- 8bW. A. Herrmann, Angew. Chem. 2002, 114, 1342–1363;
Angew. Chem. Int. Ed. 2002, 41, 1290–1309.
10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y CAS PubMed Web of Science® Google Scholar
- 9T. Yatabe, M. A. Harbison, J. D. Brand, M. Wagner, K. Mullen, P. Samori, J. P. Rabe, J. Mater. Chem. 2000, 10, 1519–1525.
- 10
- 10aY. Shibasaki, Y. Yamamoto, Multimetallic Catalysts in Organic Synthesis, Wiley-VCH, Weinheim, 2004;
10.1002/3527603557 Google Scholar
- 10bE. K. van den Beuken, B. L. Feringa, Tetrahedron 1998, 54, 12985–13011;
- 10cJ. I. van der Vlugt, Eur. J. Inorg. Chem. 2012, 363–375;
- 10dJ. H. H. Ho, S. W. S. Choy, S. A. Macgregor, B. A. Messerle, Organometallics 2011, 30, 5978–5984;
- 10eN. D. Jones, B. R. James, Adv. Synth. Catal. 2002, 344, 1126–1134;
- 10fJ. M. Lee, Y. Na, H. Han, S. Chang, Chem. Soc. Rev. 2004, 33, 302–312.
- 11
- 11aJ. N. H. Reek, S. Arevalo, R. Van Heerbeek, P. C. J. Kamer, P. Van Leeuwen in Advances in Catalysis, Vol. 49 (Eds.: ), 2006, pp. 71–151;
- 11bB. Helms, J. M. J. Frechet, Adv. Synth. Catal. 2006, 348, 1125–1148;
- 11cD. Astruc, C. R. Chim. 2005, 8, 1101–1107.
- 12
- 12aM. J. Pouy, S. A. Delp, J. Uddin, V. M. Ramdeen, N. A. Cochrane, G. C. Fortman, T. B. Gunnoe, T. R. Cundari, M. Sabat, W. H. Myers, ACS Catal. 2012, 2, 2182–2193;
- 12bM. Katari, M. N. Rao, G. Rajaraman, P. Ghosh, Inorg. Chem. 2012, 51, 5593–5604;
- 12cL. Canovese, F. Visentin, C. Levi, C. Santo, Inorg. Chim. Acta 2012, 391, 141–149;
- 12dE. Alvarado, A. C. Badaj, T. G. Larocque, G. G. Lavoie, Chem. Eur. J. 2012, 18, 12112–12121;
- 12eS. Gaillard, J. Bosson, R. S. Ramon, P. Nun, A. M. Z. Slawin, S. P. Nolan, Chem. Eur. J. 2010, 16, 13729–13740.
- 13X. Zeng, G. D. Frey, R. Kinjo, B. Donnadieu, G. Bertrand, J. Am. Chem. Soc. 2009, 131, 8690–8696.
- 14CCDC 930168 (4) and 930169 (6) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.