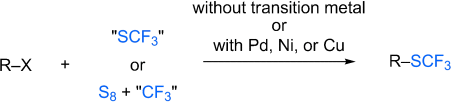Formation of CSCF3 Bonds through Direct Trifluoromethylthiolation†
T.B. thanks the CNRS and the réseau français du fluor, A.T. the Leibniz-Institut für Katalyse and Prof. Matthias Beller (LIKAT) for support.
Graphical Abstract
Modern chemistry with an old substituent: The introduction of the SCF3 group into organic substrates is a challenging task because of harsh or specific synthetic methods. However, recent advances in the formation of CSCF3 bonds include the trifluoromethylthiolation with transition-metal-free systems or in the presence of palladium, nickel, or copper catalysts (see scheme).