Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: Neurotrophic Natural Products: Chemistry and Biology (Angew. Chem. Int. Ed. 4/2014)
- Page: 893
- First Published: 21 January 2014

The human brain is an incredibly complex network of neurons that controls life, thoughts, and emotion. Age- or disease-related neural degeneration leads to breakdown of this network, incapacitating the affected individual. Certain natural products can induce outgrowth of neurites, the bridges that allow cell–cell communication in the brain, and so restore the neuronal web. E. A. Theodorakis et al. summarize in their Review on page 956 ff. recent advances in the synthesis of these molecules and highlight their potential as tools for studying neuronal web structure and function.
Inside Cover: Stimuli-Responsive Self-Assembly of a Naphthalene Diimide by Orthogonal Hydrogen Bonding and Its Coassembly with a Pyrene Derivative by a Pseudo-Intramolecular Charge-Transfer Interaction (Angew. Chem. Int. Ed. 4/2014)
- Page: 894
- First Published: 20 January 2014

A trap is set when hydrogen bonding between a supramolecular polymer with a pendant acceptor (A) and a pyrene donor (D) produces a transient supramolecular donor–acceptor complex. As A. Das and S. Ghosh show in their Communication on page 1092 ff., a charge-transfer interaction between the donor and acceptor then causes this complex to undergo back folding, transforming it into an alternate D–A stack, engulfing the pyrene donors like flies in a Venus fly trap.
Inside Back Cover: Cobalt Imidazolate Metal–Organic Frameworks Photosplit CO2 under Mild Reaction Conditions (Angew. Chem. Int. Ed. 4/2014)
- Page: 1167
- First Published: 21 January 2014

An artificial photosynthesis system is described by X. Wang and co-workers in their Communication on page 1034 ff. The porous characteristics of a metal–organic framework (MOF), for CO2 capture, were combined with the catalytic functions of imidazolate groups and cobalt to generate a cobalt-containing zeolitic imidazolate framework. By cooperating with a ruthenium-based photosensitizer, this MOF could reduce CO2 to CO with a catalytic turnover number of about 450 within 2.5 hours under mild reaction conditions.
Back Cover: Molecular Rotors in Porous Organic Frameworks (Angew. Chem. Int. Ed. 4/2014)
- Page: 1168
- First Published: 21 January 2014

Para-phenylene rings in porous aromatic frameworks rapidly flicker, resembling butterflies that flap their wings. In their Communication on page 1043 ff., P. Sozzani et al. demonstrate that these low-density, yet robust covalent architectures can sustain extremely rapid rotational motion of the phenylene rings.
Graphical Abstract
Corrigenda
Corrigendum: Fenestranes in Synthesis: Unique and Highly Inspiring Scaffolds
- Page: 911
- First Published: 21 January 2014
Corrigendum: Bis(amino)cyclopropenylidenes as Organocatalysts for Acyl Anion and Extended Umpolung Reactions
- Page: 912
- First Published: 21 January 2014
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 4/2014
- Pages: 914-917
- First Published: 21 January 2014
Author Profile
News
Bharat Ratna: C. N. R. Rao / Alwin Mittasch Special Prize: P. Jacobs / Honorary Doctorate: J. Sauer
- Page: 919
- First Published: 20 January 2014
Book Review
Palladium-Catalyzed Coupling Reactions. Practical Aspects and Future Developments. Edited by Árpád Molnár.
- Pages: 920-922
- First Published: 18 December 2013
Highlights
Alkaloid Synthesis
Synthesis of the C18-Norditerpenoid Alkaloid Neofinaconitine: A Lesson in Convergent Synthesis Planning
- Pages: 924-926
- First Published: 06 December 2013
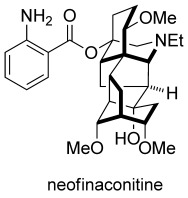
Hexacyclic framework: The total synthesis of the complex C18-norditerpenoid alkaloid neofinaconitine has been achieved by a convergent approach. This remarkable synthesis featured two Diels–Alder cycloadditions and subsequent Mannich-type N-acyliminium and radical cyclizations to establish the unique hexacyclic core structure of the target molecule.
Natural Products
Omnia praeclara rara. The Quest for Ingenol Heats Up†
- Pages: 927-929
- First Published: 25 November 2013
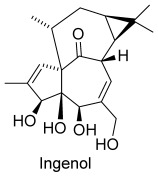
Hit the mark: The development of a short synthesis of ingenol may mark the beginning of a new era of natural products synthesis, an era where structural complexity does not deter the development of processes amenable to scale up. This may foster the exploration of biologically relevant chemical space and pave the way to the development of commercial syntheses of natural products.
Materials Science
Chirality-Driven Wettability Switching and Mass Transfer†
- Pages: 930-932
- First Published: 27 November 2013
Enantioselective wetting: Regulating the surface wettability of materials through chiral molecules provides new insight into the design of chiral materials. By taking advantage of a reversible conformational transition, smart polymers present an ideal platform for translating weak chiral signals into macroscopic properties of materials, thus resulting in a distinctive wettability switching driven by chirality (see scheme).
Correspondence
Aniline Radical Cation
Comment on “Synthesis, Characterization, and Structures of a Persistent Aniline Radical Cation”: A New Interpretation Is Necessary†
- Pages: 934-937
- First Published: 18 December 2013
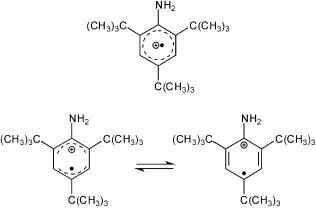
Further scrutiny for the aniline radical cation: The author of this Correspondence claims that the original spectrometric data for 2,4,6-tri-tert-butylaniline (TBA) reported in 2012 do not correspond to a stoichiometrically pure TBA.+SbF6− salt, the quantum-chemical data do not support the reported structural data, and the interpretation of the apparent temperature-dependent structural changes in the solid state is open to interpretations other than the Jahn–Teller effect.
Comment on “Synthesis, Characterization, and Structures of Persistent Aniline Radical Cation”: It Is a Protonated Aniline and Not an Aniline Radical Cation†
- Pages: 938-942
- First Published: 20 December 2013
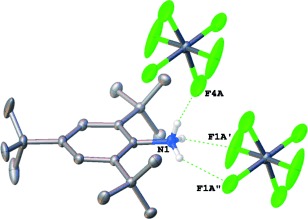
The same, but different: The reaction of tri-tert-butylaniline (TBA) with AgSbF6 in CH2Cl2 produces a green-colored intermediate which undergoes decomposition to form a protonated aniline (TBAH+SbF6−). Crystals of the protonated aniline salt (see picture) were analyzed by X-ray diffraction and found to have the same crystal characteristics as the crystals of the supposed cation radical first identified in 2012.
Reply to Comments on “Synthesis, Characterization, and Structures of Persistent Aniline Radical Cation”†
- Pages: 943-945
- First Published: 18 December 2013
The identification of the aniline radical cation TBA.+ (TBA=2,4,6-tBu3C6H2NH2) reported in 2012 was questioned in the preceeding Correspondence articles. The original authors have reexamined their UV/Vis absorption and EPR data and insist that TBA.+ radical cation is indeed persistent and stable. The possible cocrystallization of TBA.+ with TBAH+ makes crystal structures more complicated.
Minireview
Surfactants
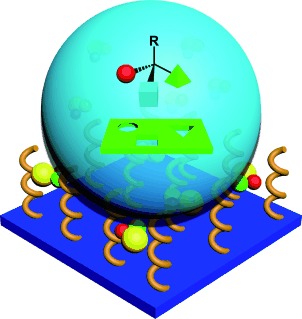
Hybrid Surfactant Systems with Inorganic Constituents
- Pages: 946-954
- First Published: 15 November 2013
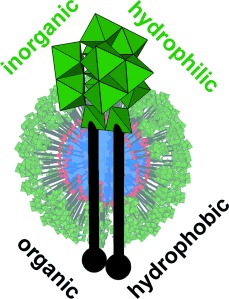
Surf′s up! Most surfactants are organic and contain a polar head group linked to a hydrophobic chain. However, the amphiphilic properties can be combined with the advanced functionality of transition-metal building blocks to give a new family of surfactants that have synergistic properties, and may lead to potential applications in catalysis, drug delivery, and smart materials.
Review
Neurotrophin
Neurotrophic Natural Products: Chemistry and Biology
- Pages: 956-987
- First Published: 18 December 2013
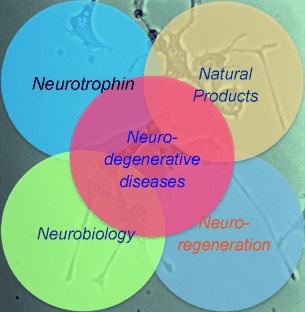
Touching a nerve: Neurotrophic natural products offer a promising therapeutic approach against various neurological disorders. This Review highlights the current synthetic strategies toward these compounds, summarizes their ability to induce neuronal growth, and discusses their potential in treating neurodegenerative diseases.
Communications
Affinity Pairs
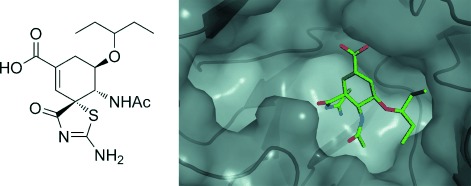
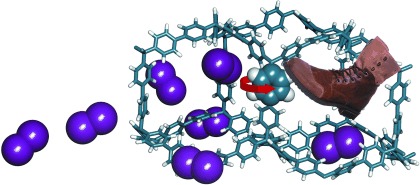
Cucurbit[7]uril⋅Guest Pair with an Attomolar Dissociation Constant†
- Pages: 988-993
- First Published: 02 January 2014
G-Quadruplex Ligands
Photo-Cross-Linking Probes for Trapping G-Quadruplex DNA†
- Pages: 994-998
- First Published: 11 December 2013
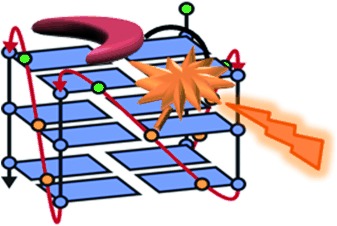
Catch me if you can: A readily accessible set of photoactivatable G-quadruplex (G4) ligands with a bisquinolinium core showed high G4-versus-duplex selectivity. Alkylation under UV/Vis irradiation occurred at G4 nucleobases located in either the loops or the external G-quartets (see picture), depending on the cross-linker and the topology of the quadruplex. These probes might be used to irreversibly trap G4 structures for the study of G4 biology.
Quadruplex–Drug Complexes
Solution Structure of a G-quadruplex Bound to the Bisquinolinium Compound Phen-DC3†
- Pages: 999-1002
- First Published: 16 December 2013
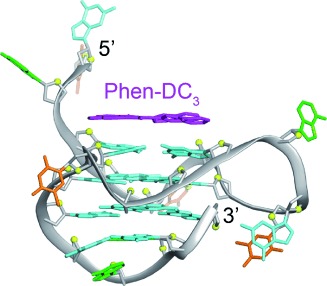
Evidence stacks up for π-stacking: The structure of the complex formed between Phen-DC3, which specifically targets G-quadruplexes and exhibits potent biological activity in vivo, and an intramolecular G-quadruplex derived from the c-myc promoter was solved by NMR spectroscopy. Phen-DC3 was found to interact with the quadruplex through extensive π-stacking with guanine bases of the top G-tetrad (see picture).
Molecular Recognition
Cucurbit[7]uril: A High-Affinity Host for Encapsulation of Amino Saccharides and Supramolecular Stabilization of Their α-Anomers in Water†
- Pages: 1003-1007
- First Published: 05 December 2013
Molecular Imaging
Multifunctional Core–Shell Silica Nanoparticles for Highly Sensitive 19F Magnetic Resonance Imaging†
- Pages: 1008-1011
- First Published: 20 January 2014
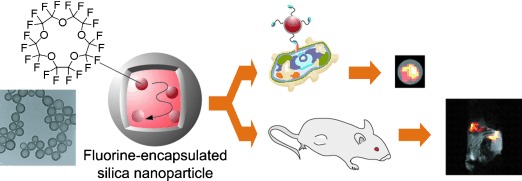
Highly sensitive 19F magnetic resonance imaging (MRI) is a useful method for in vivo imaging without background signals. However, the low sensitivity of 19F MRI limits its practical application. Novel multifunctional nanoparticles for highly sensitive 19F MRI are reported, which consist of a liquid perfluorocarbon core and a silica shell (see picture).
Drug Design
Near-Infrared Light-Mediated Photoactivation of a Platinum Antitumor Prodrug and Simultaneous Cellular Apoptosis Imaging by Upconversion-Luminescent Nanoparticles†
- Pages: 1012-1016
- First Published: 06 December 2013
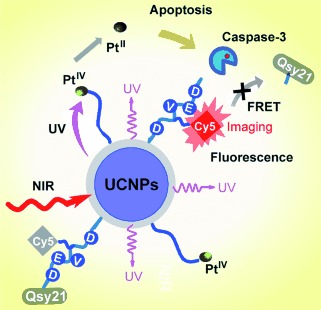
Platinum-based drugs: Near-infrared (NIR) light illumination of conjugates made of photoactive platinum(IV) prodrugs and upconversion-luminescent nanoparticles (UCNPs) is used for the remotely controlled activation of antitumor effects and for simultaneous initiation of apoptosis in the targeted tumor cells. The apoptosis-dependent caspase-3 enzyme offers the promising possibility of imaging apoptosis in real time.
Venom Peptides
Chemical Synthesis, 3D Structure, and ASIC Binding Site of the Toxin Mambalgin-2†
- Pages: 1017-1020
- First Published: 09 December 2013
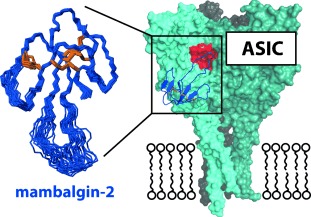
Toxic fingers pick ASIC's pocket: Efficient chemical synthesis of mambalgin-2 using native chemical ligation permitted the first structure determination of a member of this family of analgesic snake toxins. Electrophysiological analysis suggests that mambalgin-2, which was shown to adopt a three-finger toxin fold, binds near the acidic pocket on acid-sensing ion channels (ASICs).
Polycyclic Arenes
Infrared Spectra of Protonated Coronene and Its Neutral Counterpart in Solid Parahydrogen: Implications for Unidentified Interstellar Infrared Emission Bands†
- Pages: 1021-1024
- First Published: 05 December 2013
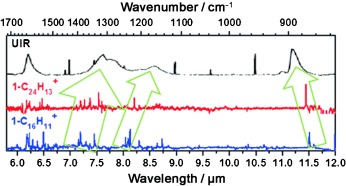
Protonated pyrene and coronene collectively appear to have the required chromophores for the unidentified infrared (UIR) emission bands from interstellar objects, and the spectral shifts on an increase in the number of aromatic rings point in the correct direction towards the positions of the UIR bands. Larger protonated peri-condensed polycyclic aromatic hydrocarbons might thus be important species among the carriers of UIR bands.
Protein Dynamics
Investigation of the Structure and Dynamics of the Capsid–Spacer Peptide 1–Nucleocapsid Fragment of the HIV-1 Gag Polyprotein by Solution NMR Spectroscopy†
- Pages: 1025-1028
- First Published: 11 December 2013

HIV-1 Gag, a retroviral polyprotein that plays a central role in viral assembly, contains several domains of known structure. Solution NMR spectroscopy, including residual dipolar couplings, heteronuclear relaxation, and backbone chemical shifts, is used to probe the conformational dynamics of the 300-residue capsid (CA)–spacer peptide 1 (SP1)–nucleocapsid (NC) fragment of Gag, with and without nucleic acids.
Photochemistry
Activation of a Photodissociative Ruthenium Complex by Triplet–Triplet Annihilation Upconversion in Liposomes†
- Pages: 1029-1033
- First Published: 11 December 2013
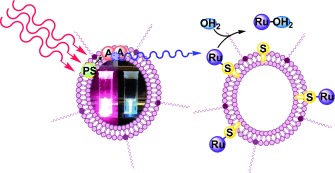
Photoactivated chemotherapy: Liposomes capable of generating blue photons in situ by triplet—triplet annihilation based upconversion of either green or red light, were prepared. The liposomes were used to trigger the photodissociation of ruthenium polypyridyl complexes from ruthenium-functionalized PEGylated liposomes upon excitation with a PDT laser at 630 nm.
Zeolites
Cobalt Imidazolate Metal–Organic Frameworks Photosplit CO2 under Mild Reaction Conditions†
- Pages: 1034-1038
- First Published: 11 December 2013
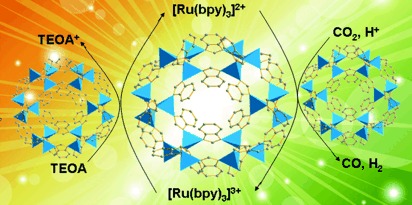
Splitting up: A cobalt-containing zeolitic imidazolate framework (Co-ZIF-9) has been used as a stable metal–organic framework cocatalyst with a photosensitizer to reduce CO2. It combines benefits of the nanoporous characteristic of Co-ZIF-9 for CO2 capture/activation and the catalytic redox function of cobalt centers. bpy=2,2′-bipyridine, TEOA=triethanolamine.
Nonporous Sorbents
Reversible and Irreversible Chemisorption in Nonporous-Crystalline Hybrids†
- Pages: 1039-1042
- First Published: 05 December 2013
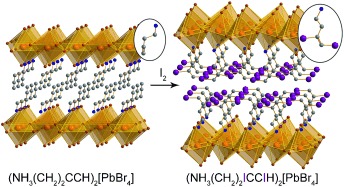
Sponges without holes: Alkyne groups in layered perovskites irreversibly capture I2 vapor with high gravimetric and volumetric capacities, accompanied by volume increases of up to 36 % with retention of crystallinity. Alkene groups in perovskites show reversible chemisorption of iodine to form diiodoalkanes, where the equilibrium for I2 release can be tuned using packing effects.
Dynamic Porous Materials
Molecular Rotors in Porous Organic Frameworks†
- Pages: 1043-1047
- First Published: 08 January 2014
Fast molecular dynamics and large sorption capacity were combined in porous organic frameworks. The low-density, yet robust covalent architectures sustain extremely rapid rotational motion of the phenylene rings up to high temperatures. Porosity enables modulation of rotor dynamics by chemical stimuli: linear alkanes and iodine vapors, pervading the material, regulate rotor speed at will.
Supramolecular Chemistry
Self-Host Blue-Emitting Iridium Dendrimer with Carbazole Dendrons: Nondoped Phosphorescent Organic Light-Emitting Diodes†
- Pages: 1048-1052
- First Published: 06 December 2013
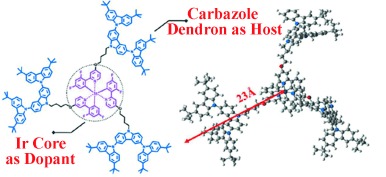
Out of the blue: The title dendrimer has been developed by covalently attaching a second-generation carbazole dendron to an emissive tris[2-(2,4-difluorophenyl)-pyridyl]iridium(III) core through a nonconjugated linker to form an efficient self-host system (see figure). Unlike small molecular phosphors and other phosphorescent dendrimers known to date, the nondoped phosphorescent organic light-emitting diodes herein are realized without a loss in efficiency.
Rare-Earth Complexes
Homometallic Rare-Earth Metal Phosphinidene Clusters: Synthesis and Reactivity†
- Pages: 1053-1056
- First Published: 05 December 2013
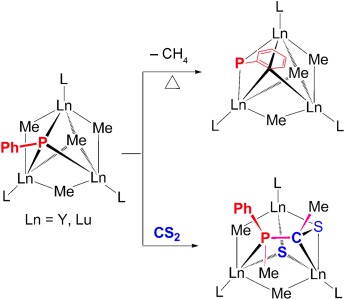
A rare gathering: Two new trinuclear μ3-bridged rare-earth metal phosphinidene complexes were synthesized by treatment of the corresponding carbene precursors with phenylphosphine; some new transformation patterns of phosphinidenes are revealed. A possible pathway for reaction of these phosphinidene complexes with CS2 was determined by DFT calculations.
Proteins
Mild Bioconjugation Through the Oxidative Coupling of ortho-Aminophenols and Anilines with Ferricyanide†
- Pages: 1057-1061
- First Published: 05 December 2013
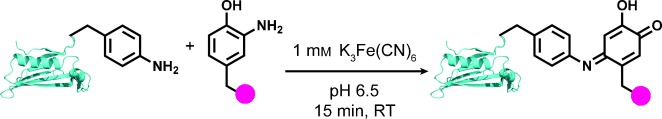
Having a complex: By using a small-molecule-based screen, ferricyanide was identified as an alternative, mild oxidant for the bioconjugation of anilines and o-aminophenols. The efficient coupling reaction is compatible with thiols and 1,2-diols, thus allowing its use in the creation of complex modified proteins.
Carbon Dispersant
The Colloidal Stabilization of Carbon with Carbon: Carbon Nanobubbles as both Dispersant and Glue for Carbon Nanotubes†
- Pages: 1062-1066
- First Published: 05 December 2013
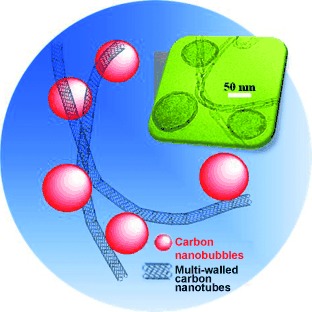
Tiny bubbles: A unique organic surfactant-free dispersion phenomenon of carbon nanostructures in aqueous solution is presented. Here, spontaneous Schottky charge transfer from carbon nanotubes (CNTs) to nitrogen-doped carbon “nanobubbles” (CNBs) occurs, creating induced charges for effective stabilization. The interaction between CNTs and CNBs can be applied to create additive-free conductive carbon devices of various forms.
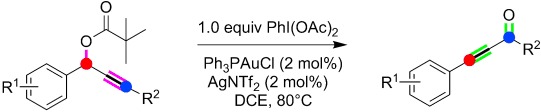
Trifluoromethylation
PhI(OAc)2-Mediated Radical Trifluoromethylation of Vinyl Azides with Me3SiCF3†
- Pages: 1067-1071
- First Published: 11 December 2013
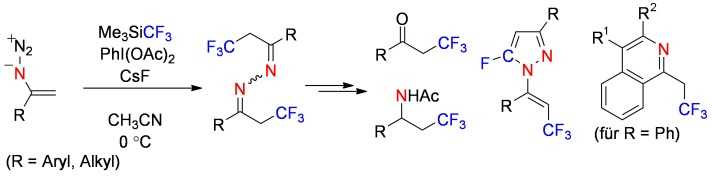
Trifluoromethylated diversity: The title reaction (see scheme) efficiently leads to α-trifluoromethyl azines, which were successfully transformed into valuable fluorine-containing molecules such as α-trifluoromethyl ketones, β-trifluoromethyl amines, 5-fluoropyrazoles, and trifluoroethyl isoquinolines.
Caged DNA
Influence of the Absolute Configuration of NPE-Caged Cytosine on DNA Single Base Pair Stability†
- Pages: 1072-1075
- First Published: 11 December 2013
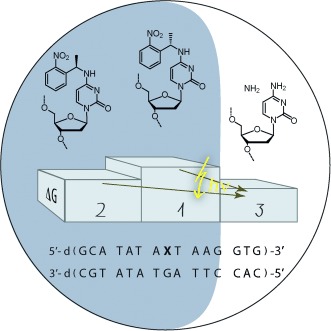
It's the stereo center: The (o-nitrophenyl)-ethyl caging group destabilizes duplex DNA. The effect depends on the absolute configuration of the stereocenter and is locally restricted. The structure models of the modified duplex DNA diastereomers reveal the distinct orientation of the caging group and provide the structural basis of the effect.
Enzyme Inhibitor
Serendipitous Discovery of a Potent Influenza Virus A Neuraminidase Inhibitor†
- Pages: 1076-1080
- First Published: 11 December 2013
H2 Activation
A d10 Ni–(H2) Adduct as an Intermediate in HH Oxidative Addition across a NiB Bond†
- Pages: 1081-1086
- First Published: 09 December 2013
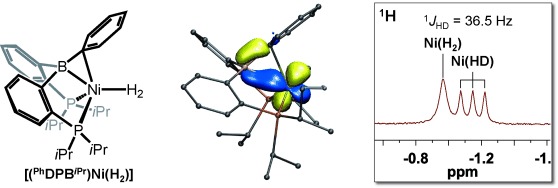
“B” for “better stability”: NMR spectroscopy of the diphosphine–borane-supported Ni–(H2) complex [(PhDPBiPr)Ni(H2)] in solution confirmed the presence of an intact H2 ligand (see picture). This nonclassical H2 adduct is an intermediate in the cooperative activation of H2 across the NiB bond. Electronic-structure calculations highlighted the important role of the borane ligand in stabilizing the Ni–(H2) interaction.
Transition States
Combined Inhibitor Free-Energy Landscape and Structural Analysis Reports on the Mannosidase Conformational Coordinate†
- Pages: 1087-1091
- First Published: 11 December 2013
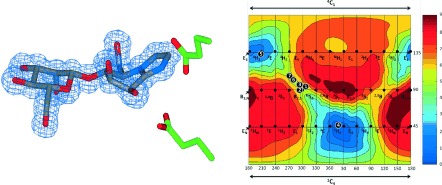
Shipshape inhibitors: Quantum mechanical calculations of the free-energy landscape (see figure) of the glycosidase transition-state mimics isofagomine and mannoimidazole reveals that only the latter is energetically poised to report upon the mannosidase transition-state conformation. X-ray structures of β-mannanases from different families reveal they both adopt a boat conformation, thus allowing unification of the enzymatic conformational itinerary of a range of diverse α- and β-mannosidases.
Supramolecular Assembly
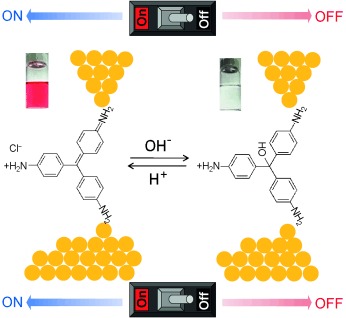
Stimuli-Responsive Self-Assembly of a Naphthalene Diimide by Orthogonal Hydrogen Bonding and Its Coassembly with a Pyrene Derivative by a Pseudo-Intramolecular Charge-Transfer Interaction†
- Pages: 1092-1097
- First Published: 27 December 2013
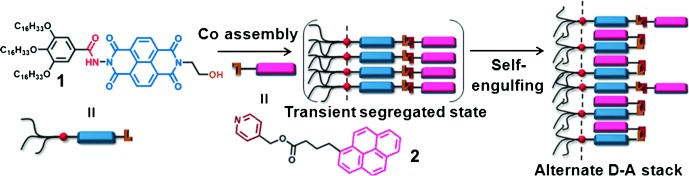
So many opportunities to get together: A naphthalene diimide building block 1 underwent self-assembly by orthogonal hydrogen bonding in a nonpolar solvent to form reverse vesicles, the temperature-dependent denaturation and redissolution of which led to reverse micelles. The coassembly of 1 with a pyrene donor 2 gave a transient donor–acceptor (D–A) complex, which formed an alternate D–A stack through a charge-transfer interaction (see picture).
Single-Molecule Devices
Single-Molecule Sensing of Environmental pH—an STM Break Junction and NEGF-DFT Approach†
- Pages: 1098-1102
- First Published: 11 December 2013
Bonding Modes
An Unusual Example of Hypervalent Silicon: A Five-Coordinate Silyl Group Bridging Two Palladium or Nickel Centers through a Nonsymmetrical Four-Center Two-Electron Bond†
- Pages: 1103-1108
- First Published: 11 December 2013

Strange to the core: In Pd and Ni dimers supported by PSiP ligands in which two hypervalent five-coordinate Si atoms bridge the two metal centers, a rare square-pyramidal geometry at Si and an unusual asymmetric M2Si2 core were observed. Natural bond orbital analysis showed that an asymmetric four-center two-electron (4c-2e) bond stabilizes the hypervalent Si atoms in the M2Si2 core (see picture).
Drug Discovery
Overcoming the Unexpected Functional Inversion of a PqsR Antagonist in Pseudomonas aeruginosa: An In Vivo Potent Antivirulence Agent Targeting pqs Quorum Sensing†
- Pages: 1109-1112
- First Published: 11 December 2013
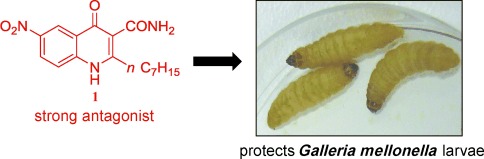
Jamming the talks for combating the bugs: The first PqsR antagonist that is potent in vivo and interrupts cell-to-cell communication in P. aeruginosa was discovered by overcoming an unexpected functional inversion that is mediated by a bacterial signal molecule synthase. The developed antagonist 1 protected G. mellonella larvae from bacterial infection at a very low dose.
Drug Delivery
Efficacious Anticancer Drug Delivery Mediated by a pH-Sensitive Self-Assembly of a Conserved Tripeptide Derived from Tyrosine Kinase NGF Receptor†
- Pages: 1113-1117
- First Published: 11 December 2013
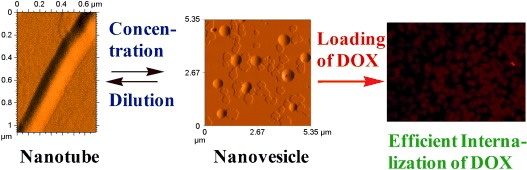
Package for special delivery: A biologically active tripeptide self-assembles to produce nanovesicles at lower concentrations and nanotubes at higher concentrations (see scheme). The nanovesicles rupture at pH≈6 and are highly efficient in doxorubicin delivery to both drug-sensitive and drug-resistant cancer cells. This system is highly promising as a stimulus-responsive biocompatible nanovehicle.
Borane Carbonyl Derivatives
Formylborane Formation with Frustrated Lewis Pair Templates†
- Pages: 1118-1121
- First Published: 11 December 2013
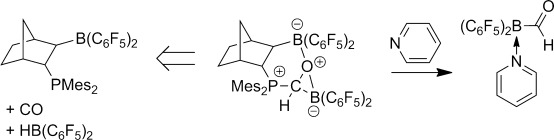
Channeling frustration into productivity: An elusive borane carbaldehyde was liberated from the product of carbon monoxide hydroboration at a frustrated Lewis pair template by treatment with pyridine and isolated as the donor-stabilized adduct (see scheme; Mes=mesityl). In this way, the thermodynamic restrictions of CO insertion into boron–hydrogen bonds could be circumvented.
Molecular Dynamics
Resonances in the Entrance Channel of the Elementary Chemical Reaction of Fluorine and Methane†
- Pages: 1122-1126
- First Published: 04 December 2013
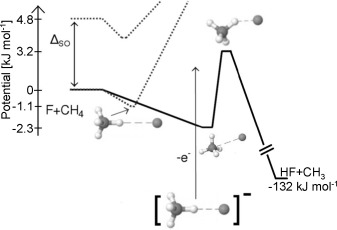
Chemical reactivity: Experimental and theoretical data consistently demonstrate the existence of resonances in the entrance channel of the F+CH4→HF+CH3 reaction shown by transition-state spectroscopy (see picture; ΔSO=atomic spin–orbit splitting). Based on full-dimensional quantum dynamics simulations, a clear picture is drawn explaining the resonances.
Ligand Design
Synthesis and Catalytic Activities of Porphyrin-Based PCP Pincer Complexes†
- Pages: 1127-1130
- First Published: 11 December 2013
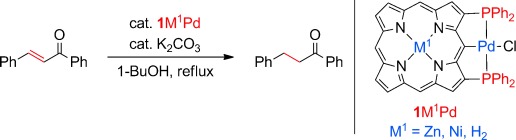
Metals at play: 2,18-Bis(diphenylphosphino)porphyrins undergo peripheral cyclometalation with group 10 transition-metal salts to afford the corresponding porphyrin-based PCP pincer complexes. The catalytic activities of the porphyrin-based pincer complexes were investigated in the allylation of benzaldehyde with allylstannane and in the 1,4-reduction of chalcone (see scheme) to assess the electronic interplay between the inner metal and the outer metal in catalysis.
The Strongest Acid
The Strongest Brønsted Acid: Protonation of Alkanes by H(CHB11F11) at Room Temperature†
- Pages: 1131-1134
- First Published: 11 December 2013
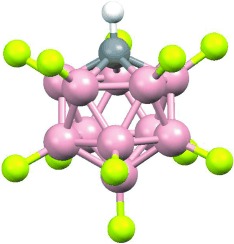
What's eating you, alkane? The fluorinated carborane acid, H(CHB11F11), is shown to be the strongest Brønsted acid presently known. Remarkably, it protonates alkanes at room temperature. Stable carbocation salts are isolated. This novel superacid provides new opportunities to study the chemistry of hydrocarbon reforming.
Cycloadditions
Rhodium(I)-Catalyzed Cyclization of Allenynes with a Carbonyl Group through Unusual Insertion of a CO Bond into a Rhodacycle Intermediate†
- Pages: 1135-1139
- First Published: 04 December 2013
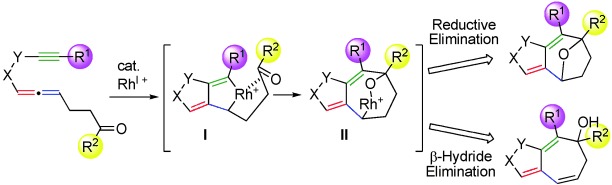
Unusual insertion: During the title reaction, a CO bond is inserted into the C(sp2)Rh bond of rhodacycle intermediate I. The insertion occurs via a highly strained transition state. Direct reductive elimination from II gives a tricyclic product containing an 8-oxabicyclo[3.2.1]octane skeleton, whereas β-hydride elimination from II gives products with fused five- and seven-membered rings.
Imaging Agents
Activation and Retention: A Magnetic Resonance Probe for the Detection of Acute Thrombosis†
- Pages: 1140-1143
- First Published: 11 December 2013
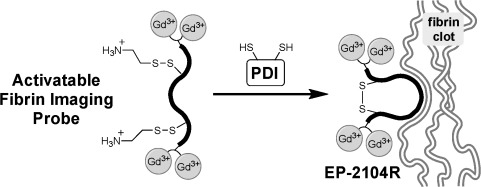
Identify new blood clots: The enzyme protein disulfide isomerase (PDI) plays an important role in facilitating the aggregation of activated platelets during the formation of new blood clots. By modifying a key disulfide bridge in the fibrin imaging probe EP-2104R, a new activatable magnetic resonance probe has been prepared that is responsive to PDI and therefore has the potential to be used for the identification of nascent blood clots.
Homogeneous Gold Catalysis
Dehydrogenative Meyer–Schuster-Like Rearrangement: A Gold-Catalyzed Reaction Generating an Alkyne†
- Pages: 1144-1147
- First Published: 11 December 2013
Gold(I)-Catalyzed Diastereoselective Hydroacylation of Terminal Alkynes with Glyoxals†
- Pages: 1148-1151
- First Published: 11 December 2013
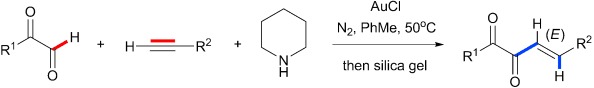
The simple AuCl catalyst and piperidine convert terminal alkynes and α-ketoaldehydes into 1,2-dicarbonyl-3-enes, the products of a formal hydroacylation of the triple bond, with excellent regio- and diastereoselectivity. There is no evidence for classical A3 coupling products, which could be expected from such a gold-catalyzed reaction of an aldehyde, an amine, and a terminal alkyne.
Enantiomer Identification
Identifying Enantiomers in Mixtures of Chiral Molecules with Broadband Microwave Spectroscopy†
- Pages: 1152-1155
- First Published: 06 December 2013
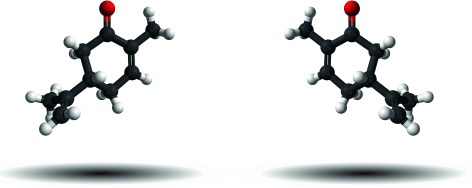
Enantiomer differentiation, enantiomeric excess measurement, and absolute configuration determination within a mixture of gas-phase chiral molecules are demonstrated. In these experiments, microwave three-wave mixing within supersonic jets is combined with chirped-pulse broadband microwave spectroscopy. This new technique is now a significant step closer to broader application.
Hydration Clusters
Direct Spectroscopic Detection of the Orientation of Free OH Groups in Methyl Lactate–(Water)1,2 Clusters: Hydration of a Chiral Hydroxy Ester†
- Pages: 1156-1159
- First Published: 04 December 2013
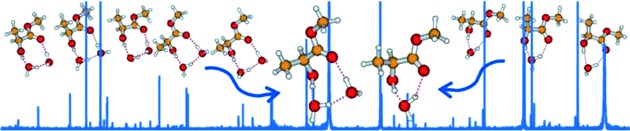
Small hydration clusters of methyl lactate show surprisingly specific binding preferences. They strongly prefer the insertion H-bonding topology, and favor specific orientation(s) for their non-H-bonded hydroxy group(s). Using a broadband chirped pulse and cavity based microwave spectroscopy, direct detection of such unique conformations of the methyl lactate–(water)1,2 clusters is possible.
Carbohydrates
Short and Sweet: D-Glucose to L-Glucose and L-Glucuronic Acid†
- Pages: 1160-1162
- First Published: 05 December 2013
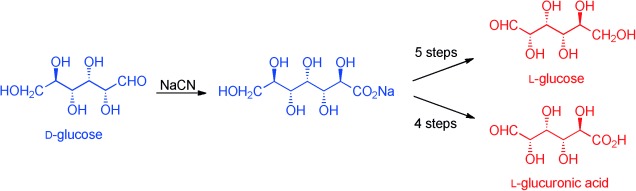
Short and sweet: The synthesis of 99.4 % pure L-glucose and L-glucuronic acid from D-glucose via very inexpensive sodium glucoheptonate requires no purification of either intermediates or final products, other than extraction into and removal of solvents; a simple crystallization will raise the purity to >99.8 %. New diacetonides of glucose and glucuronolactone are reported.
Protic N-Heterocyclic Carbenes
Oxidative Addition of 2-Halogenoazoles—Direct Synthesis of Palladium(II) Complexes Bearing Protic NH,NH-Functionalized NHC Ligands†
- Pages: 1163-1166
- First Published: 11 December 2013
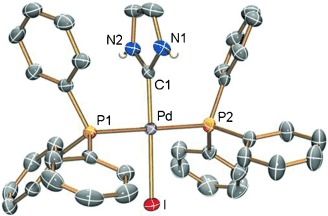
Without N-protection: The oxidative addition of neutral 2-halogenoazoles to [Pd(PPh3)4] followed by N-protonation provides access to palladium(II) complexes bearing protic NH,NH-functionalized NHC ligands (see example; NHC=N-heterocyclic carbene). The synthetic utility of the protic NH,NH-NHC ligands was demonstrated with their stepwise N,N′-alkylation.








![Cucurbit[7]uril⋅Guest Pair with an Attomolar Dissociation Constant](/cms/asset/a07baf85-17e4-4e54-8268-8de4466bc691/mcontent.jpg)
![Cucurbit[7]uril: A High-Affinity Host for Encapsulation of Amino Saccharides and Supramolecular Stabilization of Their α-Anomers in Water](/cms/asset/5808e9eb-4f65-408b-a8e4-bc072d5ae5ca/mcontent.jpg)