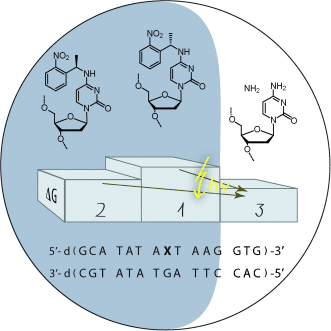
Influence of the Absolute Configuration of NPE-Caged Cytosine on DNA Single Base Pair Stability†
The work was supported by the “Schering-Stiftung” (H.S.S.) and the EU project “WeNMR” (H.R.A.J.). We thank Elke Stirnal for HPLC separation of the photocaged DNA diastereomers. Work at BMRZ is supported by the state of Hesse. H.S. and A.H. are members of the Cluster of Excellence Macromolecular Complexes. Financial support through the DFG (grant number SFB 902) is gratefully acknowledged. NPE=1-(2-nitrophenyl)ethyl.
Graphical Abstract
It's the stereo center: The (o-nitrophenyl)-ethyl caging group destabilizes duplex DNA. The effect depends on the absolute configuration of the stereocenter and is locally restricted. The structure models of the modified duplex DNA diastereomers reveal the distinct orientation of the caging group and provide the structural basis of the effect.
Abstract
Photolabile protecting groups are a versatile tool to trigger reactions by light irradiation. In this study, we have investigated the influence of the absolute configuration of the 1-(2-nitrophenyl)ethyl (NPE) cage group on a 15-base-pair duplex DNA. Using UV melting, we determined the global stability of the unmodified and the selectively (S)- and (R)-NPE-modified DNA sequences, respectively. We observe a differently destabilizing effect for the two NPE stereoisomers on the global stability. Analysis of the temperature dependence of imino proton exchange rates measured by NMR spectroscopy reveals that this effect can be attributed to decreased base pair stabilities of the caged and the 3′-neighbouring base pair, respectively. Furthermore, our NMR based structural models of the modified duplexes provide a structural basis for the distinct effect of the (S)- and the (R)-NPE group.