Serendipitous Discovery of a Potent Influenza Virus A Neuraminidase Inhibitor†
We thank the Natural Sciences and Engineering Research Council of Canada and Medical Research Council (UK) for financial support. We also thank John Skehel and Patrick Collins of the MRC-National Institute for Medical Research (UK), for the kind provision of N8 protein.
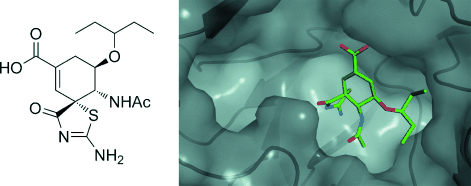
Graphical Abstract
Abstract
We have previously reported a potent neuraminidase inhibitor that comprises a carbocyclic analogue of zanamivir in which the hydrophilic glycerol side chain is replaced by the hydrophobic 3-pentyloxy group of oseltamivir. This hybrid inhibitor showed excellent inhibitory properties in the neuraminidase inhibition assay (Ki=0.46 nM; Ki (zanamivir)=0.16 nM) and in the viral replication inhibition assay in cell culture at 10−8 M. As part of this lead optimization, we now report a novel spirolactam that shows comparable inhibitory activity in the cell culture assay to that of our lead compound at 10−7 M. The compound was discovered serendipitously during the attempted synthesis of the isothiourea derivative of the original candidate. The X-ray crystal structure of the spirolactam in complex with the N8 subtype neuraminidase offers insight into the mode of inhibition.