Solution Structure of a G-quadruplex Bound to the Bisquinolinium Compound Phen-DC3†
Wan Jun Chung
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
These authors contributed equally.
Search for more papers by this authorDr. Brahim Heddi
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
These authors contributed equally.
Search for more papers by this authorDr. Florian Hamon
Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Search for more papers by this authorCorresponding Author
Dr. Marie-Paule Teulade-Fichou
Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Marie-Paule Teulade-Fichou, Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Anh Tuân Phan, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Anh Tuân Phan
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Marie-Paule Teulade-Fichou, Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Anh Tuân Phan, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorWan Jun Chung
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
These authors contributed equally.
Search for more papers by this authorDr. Brahim Heddi
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
These authors contributed equally.
Search for more papers by this authorDr. Florian Hamon
Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Search for more papers by this authorCorresponding Author
Dr. Marie-Paule Teulade-Fichou
Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Marie-Paule Teulade-Fichou, Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Anh Tuân Phan, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorCorresponding Author
Prof. Dr. Anh Tuân Phan
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Marie-Paule Teulade-Fichou, Institut Curie, Section Recherche, CNRS, UMR 176, Université Paris-Sud, Bat. 110–112, 91405 Orsay (France)
Anh Tuân Phan, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371 (Singapore)
Search for more papers by this authorThis research was supported by the Singapore Ministry of Education and Nanyang Technological University (grants to A.T.P.). Phen-DC3 is a phenanthroline dicarboxamide bisquinolinium.
Graphical Abstract
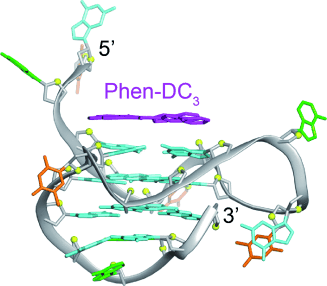
Evidence stacks up for π-stacking: The structure of the complex formed between Phen-DC3, which specifically targets G-quadruplexes and exhibits potent biological activity in vivo, and an intramolecular G-quadruplex derived from the c-myc promoter was solved by NMR spectroscopy. Phen-DC3 was found to interact with the quadruplex through extensive π-stacking with guanine bases of the top G-tetrad (see picture).
Abstract
Phen-DC3 is a highly promising compound that specifically targets G-quadruplexes, with potent biological effects observed in vivo. We used NMR spectroscopy to solve the structure of the complex formed between Phen-DC3 and an intramolecular G-quadruplex derived from the c-myc promoter. Structural information revealed that Phen-DC3 interacts with the quadruplex through extensive π-stacking with guanine bases of the top G-tetrad. On the basis of our structure, modifications are proposed for the development of this compound for selective targeting of a specific G-quadruplex conformation.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201308063_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1J. T. Davis, Angew. Chem. 2004, 116, 684–716;
10.1002/ange.200300589 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 668–698.
- 2N. V. Hud, J. Plavec in Quadruplex Nucleic Acids, Vol. 7 (Eds.: ), RSC Biomolecular Sciences, Cambridge, 2006, pp. 100–130.
- 3D. J. Patel, A. T. Phan, V. Kuryavyi, Nucleic Acids Res. 2007, 35, 7429–7455.
- 4S. Neidle, FEBS J. 2010, 277, 1118–1125.
- 5S. Balasubramanian, L. H. Hurley, S. Neidle, Nat. Rev. Drug Discovery 2011, 10, 261–275.
- 6A. Bugaut, S. Balasubramanian, Nucleic Acids Res. 2012, 40, 4727–4741.
- 7D. Monchaud, M.-P. Teulade-Fichou, Org. Biomol. Chem. 2008, 6, 627–636.
- 8K. Shin-ya, K. Wierzba, K. Matsuo, T. Ohtani, Y. Yamada, K. Furihata, Y. Hayakawa, H. Seto, J. Am. Chem. Soc. 2001, 123, 1262–1263.
- 9M. Tera, H. Ishizuka, M. Takagi, M. Suganuma, K. Shin-ya, K. Nagasawa, Angew. Chem. 2008, 120, 5639–5642;
10.1002/ange.200801235 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 5557–5560.
- 10R. Kieltyka, P. Englebienne, J. Fakhoury, C. Autexier, N. Moitessier, H. F. Sleiman, J. Am. Chem. Soc. 2008, 130, 10040–10041.
- 11I. M. Dixon, F. Lopez, A. M. Tejera, J.-P. Estève, M. A. Blasco, G. Pratviel, B. Meunier, J. Am. Chem. Soc. 2007, 129, 1502–1503.
- 12S. N. Georgiades, N. H. Abd Karim, K. Suntharalingam, R. Vilar, Angew. Chem. 2010, 122, 4114–4128;
10.1002/ange.200906363 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 4020–4034.
- 13S. G. Rzuczek, D. S. Pilch, A. Liu, L. Liu, E. J. LaVoie, J. E. Rice, J. Med. Chem. 2010, 53, 3632–3644.
- 14K. Shinohara, Y. Sannohe, S. Kaieda, K. Tanaka, H. Osuga, H. Tahara, Y. Xu, T. Kawase, T. Bando, H. Sugiyama, J. Am. Chem. Soc. 2010, 132, 3778–3782.
- 15O. P. Cetinkol, A. E. Engelhart, R. K. Nanjunda, W. D. Wilson, N. V. Hud, ChemBioChem 2008, 9, 1889–1892.
- 16S. Cosconati, L. Marinelli, R. Trotta, A. Virno, S. De Tito, R. Romagnoli, B. Pagano, V. Limongelli, C. Giancola, P. G. Baraldi, L. Mayol, E. Novellino, A. Randazzo, J. Am. Chem. Soc. 2010, 132, 6425–6433.
- 17J. Alzeer, B. R. Vummidi, P. J. Roth, N. W. Luedtke, Angew. Chem. 2009, 121, 9526–9529; Angew. Chem. Int. Ed. 2009, 48, 9362–9365.
- 18A. De Cian, E. DeLemos, J. Mergny, M.-P. Teulade-Fichou, D. Monchaud, J. Am. Chem. Soc. 2007, 129, 1856–1857.
- 19D. Monchaud, C. Allain, H. Bertrand, N. Smargiasso, F. Rosu, V. Gabelica, A. De Cian, J. L. Mergny, M.-P. Teulade-Fichou, Biochimie 2008, 90, 1207–1223.
- 20A. Piazza, J. B. Boulé, J. Lopes, K. Mingo, E. Largy, M.-P. Teulade-Fichou, A. Nicolas, Nucleic Acids Res. 2010, 38, 4337–4348.
- 21D. Gomez, A. Guédin, J. L. Mergny, B. Salles, J. F. Riou, M.-P. Teulade-Fichou, P. Calsou, Nucleic Acids Res. 2010, 38, 7187–7198.
- 22J. S. Smith, Q. Chen, L. A. Yatsunyk, J. M. Nicoludis, M. S. Garcia, R. Kranaster, S. Balasubramanian, D. Monchaud, M.-P. Teulade-Fichou, L. Abramowitz, D. C. Schultz, F. B. Johnson, Nat. Struct. Mol. Biol. 2011, 18, 478–485.
- 23J. Lopes, A. Piazza, R. Bermejo, B. Kriegsman, A. Colosio, M.-P. Teulade-Fichou, M. Fioiani, A. Nicolas, EMBO J. 2011, 30, 4033–4046.
- 24J. L. Mergny, Nat. Chem. Biol. 2012, 8, 225–226.
- 25K. Halder, E. Largy, M. Benzler, M.-P. Teulade-Fichou, J. S. Hartig, ChemBioChem 2011, 12, 1663–1668.
- 26R. Halder, J. F. Riou, M. P. Teulade-Fichou, T. Frickey, J. S. Hartig, BMC Res. Notes 2012, 5, 138.
- 27C. E. Nesbit, J. M. Tersak, E. V. Prochownik, Oncogene 1999, 18, 3004–3016.
- 28A. Siddiqui-Jain, C. L. Grand, D. J. Bearss, L. H. Hurley, Proc. Natl. Acad. Sci. USA 2002, 99, 11593–11598.
- 29A. T. Phan, V. Kuryavyi, H. Y. Gaw, D. J. Patel, Nat. Chem. Biol. 2005, 1, 167–173.
- 30A. T. Phan, Y. S. Modi, D. J. Patel, J. Am. Chem. Soc. 2004, 126, 8710–8716.
- 31A. Ambrus, D. Chen, J. Dai, R. A. Jones, D. Yang, Biochemistry 2005, 44, 2048–2058.
- 32X. Huang, P. Yu, E. LeProust, X. Gao, Nucleic Acids Res. 1997, 25, 4758–4763.
- 33N. Campbell, G. W. Collie, S. Neidle, Curr. Protoc. Nucleic Acid Chem. 2012, 50, 17.6.1–17.6.22.
- 34J. M. Nicoludis, S. T. Miller, P. D. Jeffrey, S. P. Barrett, P. R. Rablen, T. J. Lawton, L. A. Yatsunyk, J. Am. Chem. Soc. 2012, 134, 20446–20456.
- 35E. Gavathiotis, R. A. Heald, M. F. Stevens, M. S. Searle, J. Mol. Biol. 2003, 334, 25–36.
- 36C. Hounsou, L. Guittat, D. Monchaud, M. Jourdan, N. Saettel, J.-L. Mergny, M.-P. Teulade-Fichou, ChemMedChem 2007, 2, 655–666.
- 37J. Dai, M. Carver, L. H. Hurley, D. Yang, J. Am. Chem. Soc. 2011, 133, 17673–17680.
- 38C. Bazzicalupi, M. Ferraroni, A. R. Bilia, F. Scheggi, P. Gratteri, Nucleic Acids Res. 2013, 41, 632–638.
- 39W. J. Chung, B. Heddi, M. Tera, K. Iida, K. Nagasawa, A. T. Phan, J. Am. Chem. Soc. 2013, 135, 13495–13501.
- 40Transient formation of the A3⋅A12 base pair above Phen-DC3 was observed during molecular-dynamics simulations.
- 41M. C. Nielsen, J. Borch, T. Ulven, Bioorg. Med. Chem. 2009, 17, 8241–8246.
- 42T. Lemarteleur, D. Gomez, R. Paterski, E. Mandine, P. Mailliet, J.-F. Riou, Biochem. Biophys. Res. Commun. 2004, 323, 802–808.
- 43R. Rodriguez, S. Müller, J. A. Yeoman, C. Trentesaux, J.-F. Riou, S. Balasubramanian, J. Am. Chem. Soc. 2008, 130, 15758–15759.
- 44V. Dhamodharan, S. Harikrishna, C. Jagadeeswaran, K. Halder, P. I. Pradeepkumar, J. Org. Chem. 2011, 76, 229–242.
- 45C. Granotier, G. Pennarun, L. Riou, F. Hoffschir, L. R. Gauthier, A. D. Cian, D. Gomez, E. Mandine, J. F. Riou, J. L. Mergny, P. Mailliet, B. Dutrillaux, F. D. Boussin, Nucleic Acids Res. 2005, 33, 4182–4190.
- 46A. F. Larsen, M. C. Nielsen, T. Ulven, Chem. Eur. J. 2012, 18, 10892–10902.
- 47A. Hittinger, T. Caulfield, P. Mailliet, H. Bouchard, E. Mandine, J.-L. Mergny, L. Guittat, J.-F. Riou, D. Gomez, C. Belmokhtar (Aventis Pharma S.A.), WO2004072027, 2004.
- 48M. Di Antonio, G. Biffi, A. Mariani, E. A. Raiber, R. Rodriguez, S. Balasubramanian, Angew. Chem. 2012, 124, 11235–11240;
10.1002/ange.201206281 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 11073–11078.