Reversible and Irreversible Chemisorption in Nonporous-Crystalline Hybrids†
This research was funded by the Precourt Institute for Energy and the Stinehart/Reed Awards Fund. We thank Stanford University for generous start-up funds and the Stanford Gabilan Fund for partial support of D.S. Single-crystal and powder X-ray diffraction studies were performed at the Stanford Nanocharacterization Laboratory (SNL), part of the Stanford Nano Shared Facilities. We also thank M. Gembicky for assistance with crystallography, and Profs. J. Du Bois, R. M. Waymouth, and J. I. Brauman for helpful discussions.
Graphical Abstract
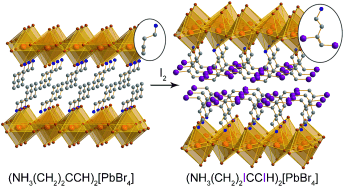
Sponges without holes: Alkyne groups in layered perovskites irreversibly capture I2 vapor with high gravimetric and volumetric capacities, accompanied by volume increases of up to 36 % with retention of crystallinity. Alkene groups in perovskites show reversible chemisorption of iodine to form diiodoalkanes, where the equilibrium for I2 release can be tuned using packing effects.
Abstract
The tools of synthetic chemistry allow us to fine-tune the reactivity of molecules at a level of precision not yet accessible with inorganic solids. We have investigated hybrids that couple molecules to the superior thermal and mechanical properties of solids. Herein we present, to the best of our knowledge, the first demonstration of reactivity between hybrid perovskites and substrates. Reaction with iodine vapor results in a remarkable expansion of these materials (up to 36 % in volume) where new covalent CI bonds are formed with retention of crystallinity. These hybrids also show unusual examples of reversible chemisorption. Here, solid-state interactions extend the lifetime of molecules that cannot be isolated in solution. We have tuned the half-lives of the iodinated structures from 3 h to 3 days. These nonporous hybrids drive substrate capture and controlled release through chemical reactivity. We illustrate the strengths of the hybrid by considering radioactive iodine capture.