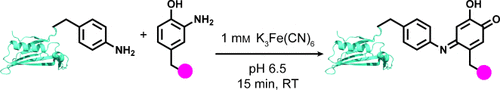Mild Bioconjugation Through the Oxidative Coupling of ortho-Aminophenols and Anilines with Ferricyanide†
Allie C. Obermeyer
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorJohn B. Jarman
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorChawita Netirojjanakul
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorKareem El Muslemany
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthew B. Francis
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720 (USA)
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)Search for more papers by this authorAllie C. Obermeyer
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorJohn B. Jarman
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorChawita Netirojjanakul
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorKareem El Muslemany
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Search for more papers by this authorCorresponding Author
Prof. Dr. Matthew B. Francis
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)
Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720 (USA)
Department of Chemistry, University of California, Berkeley, Berkeley, CA 94720-1460 (USA)Search for more papers by this authorThis work was supported by a grant from the NSF (CHE 1059083). A.C.O was supported by an NSF graduate research fellowship and the UC Berkeley Chemical Biology Program (NRSA Training Grant 1 T32 GMO66698). J.B.J. was supported by a SURF Rose-Hills summer research fellowship, and C.N. was supported by an HHMI International Student Fellowship.
Graphical Abstract
Having a complex: By using a small-molecule-based screen, ferricyanide was identified as an alternative, mild oxidant for the bioconjugation of anilines and o-aminophenols. The efficient coupling reaction is compatible with thiols and 1,2-diols, thus allowing its use in the creation of complex modified proteins.
Abstract
Using a small-molecule-based screen, ferricyanide was identified as a mild and efficient oxidant for the coupling of anilines and o-aminophenols on protein substrates. This reaction is compatible with thiols and 1,2-diols, allowing its use in the creation of complex bioconjugates for use in biotechnology and materials applications.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307386_sm_miscellaneous_information.pdf4.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1H. M. O’Hare, K. Johnsson, A. Gautier, Curr. Opin. Struct. Biol. 2007, 17, 488–494.
- 2S. Zalipsky, Bioconjugate Chem. 1995, 6, 150–165.
- 3S. C. Alley, N. M. Okeley, P. D. Senter, Curr. Opin. Chem. Biol. 2010, 14, 529–537.
- 4
- 4aW. Wu, S. C. Hsiao, Z. M. Carrico, M. B. Francis, Angew. Chem. 2009, 121, 9657–9661; Angew. Chem. Int. Ed. 2009, 48, 9493–9497;
- 4bN. Stephanopoulos, G. J. Tong, S. C. Hsiao, M. B. Francis, ACS Nano 2010, 4, 6014–6020.
- 5
- 5aJ. J. Day, B. V. Marquez, H. E. Beck, T. A. Aweda, P. D. Gawande, C. F. Meares, Curr. Opin. Chem. Biol. 2010, 14, 803–809;
- 5bG. J. Tong, S. C. Hsiao, Z. M. Carrico, M. B. Francis, J. Am. Chem. Soc. 2009, 131, 11174–11178.
- 6
- 6aE. Baslé, N. Joubert, M. Pucheault, Chem. Biol. 2010, 17, 213–227;
- 6bE. M. Sletten, C. R. Bertozzi, Angew. Chem. 2009, 121, 7108–7133;
10.1002/ange.200900942 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6974–6998;
- 6cC. H. Kim, J. Y. Axup, P. G. Schultz, Curr. Opin. Chem. Biol. 2013, 17, 412–419;
- 6dM. F. Debets, J. C. M. van Hest, F. P. J. T. Rutjes, Org. Biomol. Chem. 2013, 11, 6439–6455;
- 6eY. Takaoka, A. Ojida, I. Hamachi, Angew. Chem. 2013, 125, 4182–4200;
10.1002/ange.201207089 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 4088–4106.
- 7
- 7aM. W. Crankshaw, G. A. Grant in Current Protocols in Protein Science (Eds.: ), Wiley, New York, 2001, pp. 15.1.1–15.1.18;
- 7bJ. M. Chalker, G. J. L. Bernardes, Y. A. Lin, B. G. Davis, Chem. Asian J. 2009, 4, 630–640.
- 8H. C. Hang, C. Yu, D. L. Kato, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 2003, 100, 14846–14851.
- 9
- 9aV. W. Cornish, K. M. Hahn, P. G. Schultz, J. Am. Chem. Soc. 1996, 118, 8150–8151;
- 9bA. Dirksen, P. E. Dawson, Bioconjugate Chem. 2008, 19, 2543–2548.
- 10
- 10aK. L. Kiick, E. Saxon, D. A. Tirrell, C. R. Bertozzi, Proc. Natl. Acad. Sci. USA 2002, 99, 19–24;
- 10bJ. C. Jewett, C. R. Bertozzi, Chem. Soc. Rev. 2010, 39, 1272–1279.
- 11
- 11aM. L. Blackman, M. Royzen, J. M. Fox, J. Am. Chem. Soc. 2008, 130, 13518–13519;
- 11bN. K. Devaraj, R. Weissleder, S. A. Hilderbrand, Bioconjugate Chem. 2008, 19, 2297–2299.
- 12N. Li, R. K. V. Lim, S. Edwardraja, Q. Lin, J. Am. Chem. Soc. 2011, 133, 15316–15319.
- 13C. R. Behrens, J. M. Hooker, A. C. Obermeyer, D. W. Romanini, E. M. Katz, M. B. Francis, J. Am. Chem. Soc. 2011, 133, 16398–16401.
- 14L. S. Witus, M. B. Francis, Acc. Chem. Res. 2011, 44, 774–783.
- 15
- 15aR. A. Miller, A. D. Presley, M. B. Francis, J. Am. Chem. Soc. 2007, 129, 3104–3109;
- 15bM. T. Dedeo, K. E. Duderstadt, J. M. Berger, M. B. Francis, Nano Lett. 2010, 10, 181–186.
- 16A. P. Esser-Kahn, A. T. Iavarone, M. B. Francis, J. Am. Chem. Soc. 2008, 130, 15820–15822.
- 17
- 17aJ. M. Hooker, E. W. Kovacs, M. B. Francis, J. Am. Chem. Soc. 2004, 126, 3718–3719;
- 17bJ. M. Hooker, A. P. Esser-Kahn, M. B. Francis, J. Am. Chem. Soc. 2006, 128, 15558–15559.
- 18
- 18aL. Wang, A. Brock, B. Herberich, P. G. Schultz, Science 2001, 292, 498–500;
- 18bR. A. Mehl, J. C. Anderson, S. W. Santoro, L. Wang, A. B. Martin, D. S. King, D. M. Horn, P. G. Schultz, J. Am. Chem. Soc. 2003, 125, 935–939;
- 18cZ. M. Carrico, D. W. Romanini, R. A. Mehl, M. B. Francis, Chem. Commun. 2008, 1205.
- 19
- 19aJ. H. Strauss, Jr., R. L. Sinsheimer, J. Mol. Biol. 1963, 7, 43–54;
- 19bK. Valegård, L. Liljas, K. Fridborg, T. Unge, Nature 1990, 345, 36–41.
- 20
- 20aE. Antonini, M. Brunori, J. Wyman, Biochemistry 1965, 4, 545–551;
- 20bJ. F. Corbett, J. Chem. Soc. B 1969, 207–212;
- 20cS. Gardlik, K. V. Rajagopalan, J. Biol. Chem. 1991, 266, 4889–4895;
- 20dY. Yamagishi, H. Ashigai, Y. Goto, H. Murakami, H. Suga, ChemBioChem 2009, 10, 1469–1472.
- 21
- 21aL. P. Hadjipetrou, T. Gray-Young, M. D. Lilly, J. Gen. Microbiol. 1966, 45, 479–488;
- 21bC. Liu, T. Sun, Y. Zhai, S. Dong, Talanta 2009, 78, 613–617.
- 22
- 22aS. C. Alley, K. E. Anderson, Curr. Opin. Chem. Biol. 2013, 17, 406–411;
- 22bJ. Caravella, A. Lugovskoy, Curr. Opin. Chem. Biol. 2010, 14, 520–528.
- 23J. K. Hamilton, F. Smith, J. Am. Chem. Soc. 1956, 78, 5910–5912.
- 24C. Netirojjanakul, L. S. Witus, C. R. Behrens, C.-H. Weng, A. T. Iavarone, M. B. Francis, Chem. Sci. 2013, 4, 266–272.
- 25
- 25aJ. M. Gilmore, R. A. Scheck, A. P. Esser-Kahn, N. S. Joshi, M. B. Francis, Angew. Chem. 2006, 118, 5433–5437;
10.1002/ange.200600368 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 5307–5311;
- 25bL. S. Witus, T. Moore, B. W. Thuronyi, A. P. Esser-Kahn, R. A. Scheck, A. T. Iavarone, M. B. Francis, J. Am. Chem. Soc. 2010, 132, 16812–16817.