Direct Spectroscopic Detection of the Orientation of Free OH Groups in Methyl Lactate–(Water)1,2 Clusters: Hydration of a Chiral Hydroxy Ester†
This research was funded by the University of Alberta and the Natural Sciences and Engineering Research Council (NSERC) of Canada. We also gratefully acknowledge access to the computing facilities of the Shared Hierarchical Academic Research Computing Network (SHARCNET: www.sharcnet.ca), the Western Canada Research Grid (Westgrid), and Compute/Calcul Canada. Y.X. and W.J. are holders of Canada Research Chairs (Tier I).
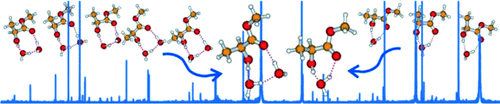
Graphical Abstract
Small hydration clusters of methyl lactate show surprisingly specific binding preferences. They strongly prefer the insertion H-bonding topology, and favor specific orientation(s) for their non-H-bonded hydroxy group(s). Using a broadband chirped pulse and cavity based microwave spectroscopy, direct detection of such unique conformations of the methyl lactate–(water)1,2 clusters is possible.
Abstract
Hydration of chiral molecules is a subject of significant current interest in light of recent experimental observations of chirality transfer from chiral solutes to water in solution and the important roles which water plays in biological events. Using a broadband chirped pulse and a cavity based microwave spectrometer, we detected spectroscopic signatures of the mono- and dihydrates of methyl lactate, a chiral hydroxy ester. Surprisingly, these small hydration clusters show highly specific binding preferences. Not only do they strongly prefer the insertion H-bonding topology, but they also favor specific pointing direction(s) for their non-H-bonded hydroxy group(s). We observed that the particular dihydrate conformer identified is not the most stable one predicted. This work highlights the superior capability of high-resolution spectroscopy to identify specific water binding topologies, and provides quantitative data to test state-of-the-art theory.