Formylborane Formation with Frustrated Lewis Pair Templates†
Dr. Muhammad Sajid
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
X-ray crystal-structure analysis.
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)Search for more papers by this authorDr. Muhammad Sajid
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Search for more papers by this authorDr. Gerald Kehr
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Search for more papers by this authorDr. Constantin G. Daniliuc
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
X-ray crystal-structure analysis.
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerhard Erker
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)
Organisch-Chemisches Institut der Universität Münster, Corrensstrasse 40, 48149 Münster (Germany)Search for more papers by this authorFinancial support from the European Research Council is gratefully acknowledged.
Graphical Abstract
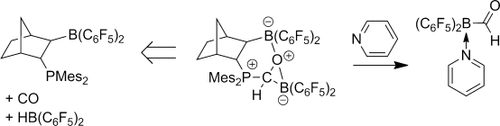
Channeling frustration into productivity: An elusive borane carbaldehyde was liberated from the product of carbon monoxide hydroboration at a frustrated Lewis pair template by treatment with pyridine and isolated as the donor-stabilized adduct (see scheme; Mes=mesityl). In this way, the thermodynamic restrictions of CO insertion into boron–hydrogen bonds could be circumvented.
Abstract
Boranes R2BH react with carbon monoxide by forming the respective borane carbonyl compounds R2BH(CO). The formation of (C6F5)2BH(CO) derived from the Piers borane, HB(C6F5)2, is a typical example. Subsequent CO-hydroboration does not take place, since the formation of the formylborane is usually endothermic. However, an “η2-formylborane” was formed by CO-hydroboration with the Piers borane at vicinal phosphane/borane frustrated Lewis pair (FLP) templates. Subsequent treatment with pyridine liberated the intact formylborane from the FLP framework, and (pyridine)(C6F5)2BCHO was then isolated as a stable compound. This product underwent typical reactions of carbonyl compounds, such as Wittig olefination.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
| Filename | Description |
|---|---|
| anie_201307551_sm_miscellaneous_information.pdf1.5 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1A. B. Burg, H. I. Schlesinger, J. Am. Chem. Soc. 1937, 59, 780–787.
- 2S. H. Bauer, J. Am. Chem. Soc. 1937, 59, 1804–1812.
- 3W. Gordy, H. Ring, A. B. Burg, Phys. Rev. 1950, 78, 512–517.
- 4R. D. Cowan, J. Chem. Phys. 1950, 18, 1101–1107.
- 5G. W. Bethke, M. K. Wilson, J. Chem. Phys. 1957, 26, 1118–1130.
- 6For a metal-stabilized formylcarborane system, see: S. Anderson, J. C. Jeffery, Y. H. Liao, D. F. Mullica, E. L. Sappenfield, F. G. A. Stone, Organometallics 1997, 16, 958–971.
- 7For rare examples of Lewis acid stabilized formylborate systems, see:
- 7aA. Berkefeld, W. E. Piers, M. Parvez, L. Castro, L. Maron, O. Eisenstein, J. Am. Chem. Soc. 2012, 134, 10843–10851;
- 7bR. Dobrovetsky, D. W. Stephan, J. Am. Chem. Soc. 2013, 135, 4974–4977.
- 8M. Sajid, L. M. Elmer, C. Rosorius, C. G. Daniliuc, S. Grimme, G. Kehr, G. Erker, Angew. Chem. 2013, 125, 2299–2302;
10.1002/ange.201208750 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 2243–2246.
- 9M. Sajid, G. Kehr, T. Wiegand, H. Eckert, C. Schwickert, R. Pöttgen, A. J. P. Cardenas, T. H. Warren, R. Fröhlich, C. G. Daniliuc, G. Erker, J. Am. Chem. Soc. 2013, 135, 8882–8895.
- 10P. Spies, G. Erker, G. Kehr, K. Bergander, R. Fröhlich, S. Grimme, D. W. Stephan, Chem. Commun. 2007, 5072–5074.
- 11
- 11aD. W. Stephan, G. Erker, Angew. Chem. 2010, 122, 50–81; Angew. Chem. Int. Ed. 2010, 49, 46–76;
- 11b“Frustrated Lewis Pairs I”: G. Erker, D. W. Stephan, Topics in Current Chemistry, Springer, Berlin, 2013, p. 332;
- 11c“Frustrated Lewis Pairs II”: G. Erker, D. W. Stephan, Topics in Current Chemistry, Springer, Berlin, 2013, p. 334.
- 12G. Kehr, S. Schwendemann, G. Erker, Top. Curr. Chem. 2013, 320-331, 45–84.
- 13See Ref. [7b] for comparison.
- 14L. J. Hounjet, C. Bannwarth, C. N. Garon, C. B. Caputo, S. Grimme, D. W. Stephan, Angew. Chem. 2013, 125, 7640–7643;
10.1002/ange.201303166 Google ScholarAngew. Chem. Int. Ed. 2013, 52, 7492–7495.
- 15
- 15aD. J. Parks, R. E. von H. Spence, W. E. Piers, Angew. Chem. 1995, 107, 895–897;
10.1002/ange.19951070724 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 809–811;
- 15bW. E. Piers, T. Chivers, Chem. Soc. Rev. 1997, 26, 345–353;
- 15cD. J. Parks, W. E. Piers, G. P. A. Yap, Organometallics 1998, 17, 5492–5503.
- 16See for comparison:
- 16aG. Schmid, H. Nöth, Chem. Ber. 1968, 101, 2502–2505;
- 16bS. Anderson, J. C. Jeffery, Y. H. Liao, D. F. Mullica, E. L. Sappenfield, F. G. A. Stone, Organometallics 1997, 16, 958–971;
- 16cM. Yamashita, Y. Suzuki, Y. Segawa, K. Nozaki, J. Am. Chem. Soc. 2007, 129, 9570–9571;
- 16dM. Yamashita, K. Nozaki, Bull. Chem. Soc. Jpn. 2008, 81, 1377–1392.
- 17See for comparison:
- 17aA. Terheiden, E. Bernhardt, H. Willner, F. Aubke, Angew. Chem. 2002, 114, 823–825;
10.1002/1521-3757(20020301)114:5<823::AID-ANGE823>3.0.CO;2-J Google ScholarAngew. Chem. Int. Ed. 2002, 41, 799–801;10.1002/1521-3773(20020301)41:5<799::AID-ANIE799>3.0.CO;2-S CAS PubMed Web of Science® Google Scholar
- 17bM. Gerken, G. Pawelke, E. Bernhardt, H. Willner, Chem. Eur. J. 2010, 16, 7527–7536;
- 17cA. Fukazawa, J. L. Dutton, C. Fan, L. G. Mercier, A. Y. Houghton, Q. Wu, W. E. Piers, M. Parvez, Chem. Sci. 2012, 3, 1814–1818.
- 18See for comparison:
- 18aS. G. Shore, D. Y. Jan, L. Y. Hsu, W. L. Hsu, J. Am. Chem. Soc. 1983, 105, 5923–5924;
- 18bK. Shelly, C. B. Knobler, M. F. Hawthorne, Inorg. Chem. 1992, 31, 2889–2892;
- 18cM. A. Fox, J. A. K. Howard, J. M. Moloney, K. Wade, Chem. Commun. 1998, 2487–2488;
- 18dJ. C. Jeffery, N. C. Norman, J. A. J. Parode, P. L. Timms, Chem. Commun. 2000, 2367–2368.
- 19See also for comparison: M. Yalpani, R. Köster, J. Organomet. Chem. 1992, 434, 133–141.
- 20H. C. Brown, Acc. Chem. Res. 1969, 2, 65–72.
- 21See also for comparison: J. M. Mandriquez, D. R. McAlister, R. D. Sanner, J. E. Bercaw, J. Am. Chem. Soc. 1978, 100, 2716–2724.
- 22C. M. Mömming, E. Otten, G. Kehr, R. Fröhlich, S. Grimme, D. W. Stephan, G. Erker, Angew. Chem. 2009, 121, 6770–6773;
10.1002/ange.200901636 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 6643–6646.
- 23M. Sajid, A. Stute, A. J. P. Cardenas, B. J. Culotta, J. A. M. Hepperle, T. H. Warren, B. Schirmer, S. Grimme, A. Studer, C. G. Daniliuc, R. Fröhlich, J. L. Petersen, G. Kehr, G. Erker, J. Am. Chem. Soc. 2012, 134, 10156–10168.
- 24M. Sajid, A. Klose, B. Birkmann, L. Liang, B. Schirmer, T. Wiegand, H. Eckert, A. J. Lough, R. Fröhlich, C. G. Daniliuc, S. Grimme, D. W. Stephan, G. Erker, Chem. Sci. 2013, 4, 213–219.
- 25C. M. Mömming, G. Kehr, B. Wibbeling, R. Fröhlich, B. Schirmer, S. Grimme, G. Erker, Angew. Chem. 2010, 122, 2464–2467;
10.1002/ange.200906697 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2414–2417.
- 26CCDC 970103, 970104, 970105, 970106, and 970107 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.