Journal list menu
Export Citations
Download PDFs
Cover Picture (Angew. Chem. Int. Ed. Engl. 10/1985)
- First Published: October 1985
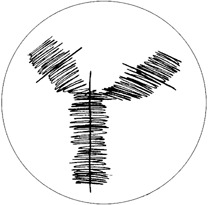
The cover shows a schematic drawing of an antibody used by Niels K. Jerne as an illustration in his Nobel Lecture. In his article the Nobel laureate points out striking analogies between linguistics and immunology. He compares the repertoire of sentences in a language with the antibody repertoire and the components of a generative grammar with the characteristic features of protein structure. Further details are found on page 810ff.
Graphical Abstract (Angew. Chem. Int. Ed. Engl. 10/1985)
- First Published: October 1985
Reviews
Solid Phase Synthesis (Nobel Lecture)†
- Pages: 799-810
- First Published: October 1985
A solid phase as “protecting group” in peptide synthesis—this was the original idea of Bruce Merrifield, who received the 1984 Nobel Prize for Chemistry. In his lecture, he describes the development of the Merrifield synthesis. In principle, all difunctional educts that may be selectively protected at one end and activated at the other can undergo reactions on solid supports.
The Generative Grammar of the Immune System (Nobel Lecture)†
- Pages: 810-816
- First Published: October 1985
The basic concepts of immunology and their development as part of biology during the last 100 years is the starting point of Niels K. Jerne's lecture, which he presented on the occasion of accepting the 1984 Nobel Prize for Physiology and Medicine. Each half of an antibody molecule consists of a light polypeptide chain containing about 214 amino acid residues and a heavy polypeptide chain containing a little more than 400 amino acid residues.
From the Structure of Antibodies to the Diversification of the Immune Responce (Noble Lecture)†
- Pages: 816-826
- First Published: October 1985
The unimaginable variety of antibody structures is evidenced by the fact that an animal produces specific antibodies against bacteria, viruses, and other foreign substances, even against substances with which it has had no prior contact. How is this possible? In his lecture, on the occasion of accepting the 1984 Nobel Prize for Physiology and Medicine, Cesar Milstein describes work directed toward answering this question. These investigations have led to, among other things, the development of the hybridoma technique in collaboration with G. Köhler.
Derivation and Diversification of Monoclonal Antibodies (Nobel Lecture)†
- Pages: 827-833
- First Published: October 1985
The trick to preparing monoclonal antibodies on a large scale consists in fusing mouse myeloma (tumor) cells with mouse spleen cells that have previously been exposed to an antigen. The hybridoma cells having the desired characteristics are immortal and secrete antibodies with a single specificity. Georges Köhler, who received the 1984 Nobel Prize for Physiology and Medicine, developed this hybridoma technique together with C. Milstein. The number of applications is legion.
Dinuclear Complexes with Predictable Magnetic Properties
- Pages: 834-850
- First Published: October 1985
The modification of the magnetic properties of a complexed transition-metalion upon formation of binuclear complexes and the magnetic behavior of binuclear complexes containing two different metals is a fascinating area of investigation. The type and strength of the interaction between the metal centers can be influenced by the choice of metal and ligand. In this way, the directed synthesis of a purely, ferromagnetic Cu2+−VO2+ complex was achieved.
Communications
Synthesis of 1,2,3-Butatrienecarboxylic Acids†
- Pages: 851-852
- First Published: October 1985
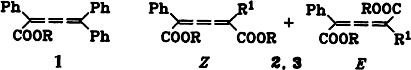
The first butatrienecarboxylic acids, the triphenyl derivative 1 and the two E/Z isomeric dicarboxylic acids 2 and 3, have been prepared. Whereas 2, R1 = tBu, is configurationally stable in solution at room temperature, 3, R1 = Ph, isomerizes under these conditions; the E form predominates in the equilibrium (R = H).
On the Thermal Behavior of Butatrienecarboxylic Acid Derivatives: Crystal and Molecular Structure of a [4]Radialene Tetracarboxylic Ester†
- Pages: 852-853
- First Published: October 1985
![On the Thermal Behavior of Butatrienecarboxylic Acid Derivatives: Crystal and Molecular Structure of a [4]Radialene Tetracarboxylic Ester](/cms/asset/20aa5f6b-2ff0-4efb-966d-5e4011671e2d/must001.jpg)
A thermal solid-state reaction with exclusive formation of the all -Z-[4]radialene 1 was observed for dimethyl (E)-1,4-diphenyl-1,2,3-butatriene-1,4-dicarboxylate. The four-membered ring in 1 is very strongly puckered; the substituents form an irregular propeller structure in which the ester and phenyl groups are twisted counter to each other.
Misinterpretation of CC Bonding through Unusually Large 2JCC Values—a Caveat for the Use of INADEQUATE-13C-NMR Spectroscopy†‡
- Pages: 854-855
- First Published: October 1985
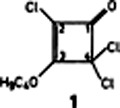
The determination of the constitution of organic compounds via 13C-13C couplings is a relatively new and useful method. Not every coupling constant greater than ca. 25 Hz, howerver, indicates the presence of a CC bond, as found in the case of chlorinated ketones, in particular, cyclobutenone derivatives such as 1. For 1, 2JC-2, C-4 is 64.1 Hz!
Diethyl Thioxomalonate S-Oxide; a Sulfine as Reactive Intermediate†‡
- Pages: 855-856
- First Published: October 1985
Do Cp(CO)2Mn Fragments Stabilize Radicals?†
- Pages: 856-858
- First Published: October 1985
Reactions in the Ligand Sphere of Iron(II): Synthesis of Crown Ethers
- Pages: 858-859
- First Published: October 1985
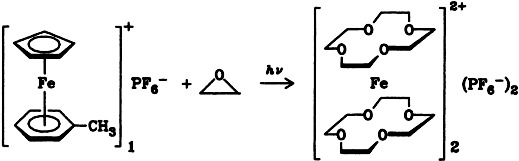
The iron sandwich complex 2 containing two [12]crown-4 ligands is formed upon irradiation of the arene-iron complexes 1 in the presence of ethyleneoxide. The Lewis acid activity of the intermediate iron complex that is photochemically formed is decisive for this specific crown ether synthesis. The structure of 2 was determined by X-ray diffraction.
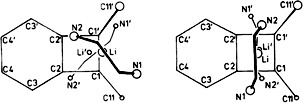
Lithium-Coordinated α-Sulfonyl Carbanions: Synthesis and X-Ray Structure Determination of [{CH2(SO2C6H5)}Li(tmeda)]2†
- Pages: 859-860
- First Published: October 1985
![Lithium-Coordinated α-Sulfonyl Carbanions: Synthesis and X-Ray Structure Determination of [{CH2(SO2C6H5)}Li(tmeda)]2](/cms/asset/cca1d611-b82b-4c43-b71c-6a80bfe363b9/must001.jpg)
In order to finally resolve the controversy over the structure of α-lithiated sulfones and α-sulfonyl carbanions, MeSO2Ph was lithiated in TMEDA and the product, the title compound, was studied by X-ray analysis. It is a dimer in which the Li, S, and O atoms form a flat eight-membered ring. The nearly planar CH2S group lies outside the coordination sphere of Li.
Novel CO Bond Formation via [3+2] Cycloaddition of Diphenylketene to a Dioxo Metal Moiety†
- Pages: 860-861
- First Published: October 1985
Skeletal Isomerization of the [Pt3(μ-PPh2)3Ph(PPh3)2] Cluster by Recrystallization in Various Solvents
- Pages: 861-862
- First Published: October 1985
The solvent used for crystallization determines which of the two structural isomers of [Pt3(μ-PPh2)3Ph(PPh3)2] 1 is formed. The isomer 1a is obtained in crystalline form from toulene/pentane, the isomer 1b · 2CH2Cl2 from CH2Cl2/pentane. The two isomers differ in their PtPt distances and PtPPh2Pt angles.
Polymorphism of an Organolithium Compound: Dilithium 1,2-diphenylbenzocyclobutadienediide · 2 Tetramethylethylenediamine†‡
- Pages: 863-864
- First Published: October 1985
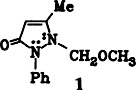
Electrochemical Methoxylation of Antipyrine†‡
- Pages: 864-865
- First Published: October 1985
[(η5-C5Me5)(CO)2MPP(2,4,6-tBu3C6H2)] (M = Fe, Ru), the First Diphosphenyl Complexes†‡
- Pages: 865-866
- First Published: October 1985
A Photochemically “Switched-on” Crown Ether Containing an Intraannular 4-Methoxyphenylazo Substituent
- Pages: 866-867
- First Published: October 1985
The binding of metal ions can be hindered by the intraannular 4-methoxyphenylazo substituent in dibenzo[18]crown-6 analogues in which a CH2OCH2 group is replaced by a (substituted) m-phenylene ring. When the substituent is photochemically flipped out of the cavity, metal ions may be complexed in the manner characteristic of crown ethers.
The First Chemical Partial Synthesis of the Nickel Complex of a Cobyrinic Acid Derivative†‡
- Pages: 867-869
- First Published: October 1985
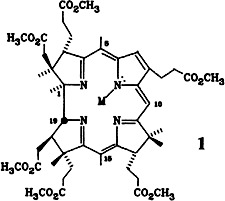
The transmetalation of a vitamin B12 building block was achieved as follows: oxidation of 1, NCo(CN)2, to the seco compound, demetalation, reaction with Ni(ClO4)2, and recyclization at 100°C to give 1, MNiClO4. Corrinoids of this kind, containing metal ions other than cobalt, were previously only accessible from natural metal-free corrinods or by total synthesis.
Cl2CClCl⊙⊕, Cl2CClBr⊙⊕, and Br2CBrCl⊙⊕ by Gas-Phase Decarbonylation of CX3COY⊙⊕†‡
- Pages: 869-870
- First Published: October 1985
The long-sought radical cation of carbon tetrachloride has been generated by decarbonylation of Cl3CCOCl⊙⊕ in the gas phase and characterized as the stable isomer Cl2CClCl⊙⊕. The theoretically predicted properties were experimentally confirmed. The dication CCl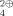 is known.
is known.
Reactions of Per(chloro, fluoro)ethanes with Aryloxide and Alkoxide Ions — Evidence for Chlorophilic Attack on CCl Bonds†
- Pages: 871-872
- First Published: October 1985
The synthesis of aryl- and alkyl per(chloro, fluoro) ethyl ethers 2 is achieved by reaction of nucleophiles ROK with the substrates 1 (X, YCl, F). This is the first confirmed example for the attack of oxygen nucleophiles on the CCl bond. The ethers 2 are only obtainable with difficulty by others routes.
Increased Production of Specific Antibodies by Presentation of the Antigen Determinants with Covalently Coupled Lipopetide Mitogens
- Pages: 872-873
- First Published: October 1985
A new concept for the production of antibodies consists in preparing a conjugate from the “lipid membrane anchor” N-palmitoyl-S[2,3-bis(palmitoyl-oxy) propyl]cysteiny1 serine and the antigen—for example, a tetradecapeptide. These conjugates are potent immunogens, which, within a few days, induce the production of high titers of antigen-specific antibodies (IgG and IgM), both in vivo and in vitro, with only one application and without either carrier proteins or Freund's adjuvant. It has already been possible to produce monoclonal antibodies with these new low-molecular-weight, chemically defined conjugates, which are obtainable via Merrifield synthesis.
Enantio- and anti-Diastereoselective Aldol Additions of Acetates and Propionates via O-Silyl Ketene Acetals†
- Pages: 874-875
- First Published: October 1985
A Previously Unrecognized 1H-Azepine Synthesis†‡
- Pages: 875-876
- First Published: October 1985
The 1H-azepine derivative 1 rather than the cyclohexadienylidenamide 3 was the species obtained by Adams and Brower in 1956, as now revealed by an X-ray analysis. Assignment of the structure on the basis of spectroscopic data was—and still is—not possible in this case. This finding opens up a simple route for the synthesis of other 2-cyano-1H-azepines.
Photochemical Synthesis of an L-Erythrose Building Block and Its use in the Preparation of Methyl 2,3,O-Isopropylidene-β-L-apio-L-furanoside†
- Pages: 877-878
- First Published: October 1985
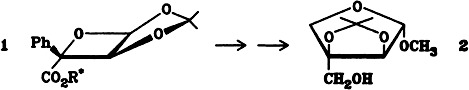
The synthesis of branched carbohydrates, e.g., the title compound 2, from nonsugar building blocks is achieved by a chirally controlled photoaldol reaction. [2+2] Cycloaddition of (−)-8-phenylmenthyl phenylglyoxylate PhCOCO2R* to 2,2-dimethyl-1,3-dioxole leads in exo fashion diastereoselectively to 1, one of four possible oxetanes; 1 is then converted in several steps into 2.
Catalytic Hydroboration with Rhodium Complexes
- Pages: 878-879
- First Published: October 1985
Polymeric Tris[trimethyltin(IV)]hexacyanocobaltate(III), a Compound Non-Analogous to “Super Prussian Blue,” and Its Tris[tricyclopentadienyluranium(IV)] Homologue†‡
- Pages: 879-881
- First Published: October 1985
The coordination polymer 1 with a three-dimensional network of Co atoms is formed as shown below. Distorted octahedral Co(CN)6 structural units are present in 1, which are linked through SnMe3 bridges such that the partial structures …CoCN → SnMe3 ← NCCo… are formed. The Sn atoms are coordinated trigonal-bipyramidally. Since, surprisingly, two-thirds of the chains are nonlinear, no super Prussian blue analogue can form; such an analogue should contain cubic Co8 cages.
Regiospecific α-Alkylation of α-Haloalkylidenamines (“α-Haloketimines”)
- Pages: 881-882
- First Published: October 1985
By masking α-haloketones 1 as α-haloalkylidenamines 2, the α-alkylation of haloketones becomes possible even when hydrogen atoms are present in the α′ position. The anions 3, obtained by the use of lithium diisopropylamide, react with alkyl halides at room temperature to form exclusively C-alkylated products 4.
Photoreactions of N-(1-Pyridinio)amidates in Monolayers and Liposomes
- Pages: 882-883
- First Published: October 1985
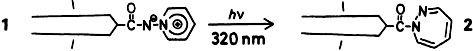
Photosensitive monolayers and liposomes can be prepared from amphiphilic pyridinioamidates such as 1 (the “double chain” symbolizes the N(CH2CH2OCOC15H31)2 residue). Their surface properties and stability can be significantly modified by UV radiation. For example, stable liposomes formed from 1 are thereby converted into metastable liposomes consisting of 1,2-diazepines 2.
Construction of Disaccharide N-Glycopeptides—Synthesis of the Linkage Region of the Transmembrance-Neuraminidase of an Influenza Virus†‡
- Pages: 883-885
- First Published: October 1985
The First [2.1] Phane: a New Helical Molecular Skeleton†
- Pages: 885-886
- First Published: October 1985
Primary Adduct, γ-Ketoketene, and Some Subsequent Products of the Reaction of Methyl 6-Oxo-5-phenyl-1,3,4-oxadiazinecarboxylate with Norbornene†
- Pages: 886-887
- First Published: October 1985
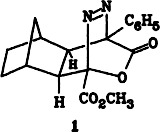
Two groups that can be easily eliminated by cycloreversion, N2 and CO2, are present in 1, the primary adduct of the title reaction. 1 could be isolated in crystalline form. Elimination of nitrogen affords a γ-ketoketene, the subsequent reactions of which indicate that it exists in equilibrium with a dihydropyrylium-2-olate.
Book Reviews
Book Review: Methods of Enzymatic Analysis. Vol. 3: Enzymes 1. Oxidoreductases, Transferases. xxiv, 605 pp., bound, DM 224.00; Vol. 4: Enzymes 2. Esterases, Glycosidases, Lyases, Ligases. xxiv, 426 pp., bound, DM 198.00; Vol. 5: Enzymes 3. Peptidases, Proteinases and Their Inhibitors. xxvii, 558 pp., bound, DM 235.00 (prices when ordering all ten volumes). Edited by H. U. Bergmeyer, J. Bergmeyer, and M. Grassl
- Pages: 888-889
- First Published: October 1985
Book Review: Polymers. Properties and Applications. Vol. 8: Polymer Degradation and Stabilization. By W. L. Hawkins
- Pages: 889-890
- First Published: October 1985
Book Review: Practical Analytical Electron Microscopy in Materials Science. By D. B. Williams
- Page: 890
- First Published: October 1985
Book Review: Beilstein Handbook of Organic Chemistry, 4th Edition, 5th Supplementary Series, Volume 17/1. Edited by R. Luckenbach
- Pages: 890-891
- First Published: October 1985
Book Review: Smectic Liquid Crystals, Textures and Structures. By G. W. Gray and J. W. Goodby
- Pages: 891-892
- First Published: October 1985





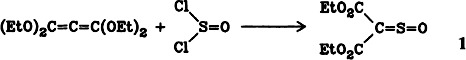
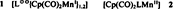
![Novel C<span class='icomoon'></span>O Bond Formation via [3+2] Cycloaddition of Diphenylketene to a Dioxo Metal Moiety](/cms/asset/1609b6dc-6cfb-47a2-9416-2b730f365068/must001.jpg)
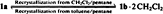
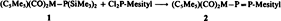


![The First [2.1] Phane: a New Helical Molecular Skeleton](/cms/asset/53f45d1b-48a1-493a-b448-50a4ac9d7bcd/must001.jpg)


































