Do Cp(CO)2Mn Fragments Stabilize Radicals?†
Dipl.-Chem. Renate Gross
Institut für Anorganische Chemie der Universität, Niederurseler Hang, D-6000 Frankfurt am Main 50 (FRG)
Search for more papers by this authorCorresponding Author
Priv.-Doz. Dr. Wolfgang Kaim
Institut für Anorganische Chemie der Universität, Niederurseler Hang, D-6000 Frankfurt am Main 50 (FRG)
Institut für Anorganische Chemie der Universität, Niederurseler Hang, D-6000 Frankfurt am Main 50 (FRG)Search for more papers by this authorDipl.-Chem. Renate Gross
Institut für Anorganische Chemie der Universität, Niederurseler Hang, D-6000 Frankfurt am Main 50 (FRG)
Search for more papers by this authorCorresponding Author
Priv.-Doz. Dr. Wolfgang Kaim
Institut für Anorganische Chemie der Universität, Niederurseler Hang, D-6000 Frankfurt am Main 50 (FRG)
Institut für Anorganische Chemie der Universität, Niederurseler Hang, D-6000 Frankfurt am Main 50 (FRG)Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft, the Fonds, der Chemischen Industrie, the Hermann-Willkomm-Stiftung, the BASF AG, the Messer Griesheim GmbH, and the Karl-Winnacker- Stiftung of Hoechst AG.
Abstract
Two classes of paramagnetic manganese complexes can be differentiated by ESR spectroscopy: radical complexes 1, in which radical-anion ligands L⊙⊖ are stabilized by Cp(CO)2Mn1 fragments, and low-spin Mn11 compounds 2, with strongly nucleophilic ligands L.
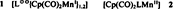
References
- 1 K. G. Caulton, Coord. Chem. Rev. 38 (1981) 1; for another example, see I.-P. Lorenz, J. Messelhäuser, W. Hiller, K. Haug, Angew. Chem. 97 (1985) 234; Angew. Chem. Int. Ed. Engl. 24 (1985) 228.
- 2 D. Sellmann, J. Muller, P. Hofmann, Angew. Chem. 94 (1982) 708; Angew. Chem. Int. Ed. Engl. 21 (1982) 691; D. Sellmann, J. Müller, J. Organoment. Chem. 281 (1985) 249.
- 3 R. Gross, W. Kaim, Angew. Chem. 96 (1984) 610; Angew. Chem. Int. Ed. Engl. 23 (1984) 614; Inorg. Chem., in press.
- 4 A. Winter, G. Huttner, L. Zsolnai, P. Kroneck, M. Gottlieb, Angew. Chem. 96 (1984) 986; Angew. Chem. Int. Ed. Engl. 23 (1984) 975; A. Winter, G. Huttner, M. Gottlieb, I. Jibril, J. Organomet. Chem. 286 (1985) 317.
- 5(a) Synthesis of radical complexes by reduction of neutral complexes with potassium in tetrahydrofuran (THF) under high vacuum. (b) [Cp1(CO)2(1)Mn]: R. Gross, W. Kaim, J. Organomet. Chem., 292 (1985) C21: (c) [(2){Cp1(CO)2Mn}2]: P. L. Gaus, N. Marchant, M. A. Marsinek, M. O. Funk, Inorg. Chem. 23 (1984) 3269; (c) [Cp1(CO)2 (3)Mn]: cf. M. Herberhold, H. Brabetz, Chem. Ber. 103 (1970) 3896, 3909.
- 6Cf. K. Wieghardt, H. Cohen, D. Meyerstein, Angew. Chem. 90 (1978) 632; Angew. Chem. Int. Ed. Engl. 17 (1978) 608.
- 7Cf. F. Gerson: Hochauflösende ESR-Spektroskopie, Verlag Chemie, Weinheim 1967, and references cited therein.
- 8 (a) Preparation of the Mn11 complexes by oxidative deprotonation of amine complexes with PbO2 in toluene [2] or TH. F. Stability and amount of synthesized Mn11 complex decrease in the series 4, 5, 6, 7 with decresing donor ability of the ligand, Isolable are [Cp1(CO)2 ( 4) Mn]: IR (THF): V(CO) = 1940, 1883 cm−1; UV (THF):λmax = 870 nm; [Cp5(CO)2 (5) Mn]: IR (THF): V(CO) = 1940, 1820 cm−1; UV (THF): λmas = 687 nm; (b) amine complexes were synthesized from photolytically generated [Cp(CO2)(thf)Mn] and the neutral ligands. [Cp1(CO)2(4-H⊕) Mn]; IR (THF): v(CO) = 1900, 1820 cm−1; UV (THF): λmax = 385 sh, 448 sh nm; [Cp5(CO)2(5-H⊕)Mn]: IR (THF):v(CO) = 1895, 1820 cm−1;(cf.[2]);[Cp1(CO)2(6-H) Mn]: IR(THF): v(CO) = 1915, 1838cm−1:UV(THF):λmax = 380 sh, 450 sh nm; 1H-NMR (C6D6): δ = 1.34(3H), 2.64 (2H, br.), 4.03 (2H), 4.26 (2H), 5.23 (2H), 7.99 (2H), each s; [Cp1 (CO)2(7-H)Mn]: IR(THF); v(CO) = 1918, 1845 cm−1; UV(THF): λmax = 375 sh, 440 sh nm; 1H-NMR (C2D6SO):δ = 1.35 (3H), 4.14 (2H), 4.48 (2H), 6.70 (1H), 7.06 (1H),7.65 (1H),12.4 (1H, br.) each s.
- 9 Arylaminyl radicals exhibit considerable spin delocalization into the aromatic π-system; F. A. Neugebauer in Landolt-Börnstein New Series, II/9c 1, p.9.
- 10(a)
F. H. Kohler,
N. Hebendanz,
U. Thewalt,
B. Kanellakopulos,
R. Klenze,
Angew. Chem.
96 (1984) 697;
10.1002/ange.19840960914 Google ScholarAngew. Chem. Int. Ed. Engl. 23 (1984) 721; (b) J. Heck, W. Massa, P. Weinig, Angew. Chem. Int. Ed. Engl. 96 (1984) 699 and Angew. Chem. Int. Ed. Engl. 23 (1984) 122.
- 11(a) M. E. Switzer, R. Wang, M. F. Rettig, A. H. Maki, J. Am. Chem. Soc. 96 (1974) 7669; (b) J. H. Ammeter, R. Bucher, N. Oswald, J. Am. Chem. Soc. 96 (1974) 7833.
- 12 J. W. Hershberger, R. J. Klingler, J. K. Kochi, J. Am. Chem. Soc. 105 (1983) 61.
- 13 CH3CN, electrolyte 0.1 M nBu4ClO4: glassy carbon working electrode vs. saturated calomel reference electrode; T = 25° C, v = 100 mV/s.
- 14 B. E. R. Schilling, R. Hoffmann, D. L. Lichtenberger, J. Am. Chem. Soc. 101 (1979) 585.
- 15 W. Kaim, Inorg. Chem. 23 (1984) 3365.
- 16 K. Krogh-Jespersen, H. J. Schugar, Inorg. Chem. 23 (1984) 4390.
- 17
For stabilization of organometal fragments in high oxidation states, see
W. A. Herrmann,
R. Serrano,
H. Bock,
Angew. Chem.
96 (1984) 365.;
10.1002/ange.19840960523 Google ScholarAngew. Chem. Int. Ed. Engl. 23 (1984) 383.
- 18 A binuclear Mn11/Mn1 compound with thiolate ligands was recently characterized at −40 °c; J. W. McDonald, Inorg. Chem. 24 (1985) 1734; the first Mn11/Mn1 complexes that are stable at room temperature can be detected in the oxidative reaction of [Cp1(CO)2 (thf)Mn] with alkoxides; R. Gross, W. Kaim, unpublished results.




