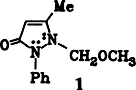
Electrochemical Methoxylation of Antipyrine†‡
Corresponding Author
Prof. Dr. Gerd Kaupp
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)Search for more papers by this authorFatih Köleli
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)
Search for more papers by this authorEleonore Gründken
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)
Search for more papers by this authorCorresponding Author
Prof. Dr. Gerd Kaupp
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)Search for more papers by this authorFatih Köleli
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)
Search for more papers by this authorEleonore Gründken
Fachbereich Chemie—Organische Chemie—der Universität, Postfach 25 03, D-2900 Oldenburg (FRG)
Search for more papers by this authorThis work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie. We thank Dr. D. Hunkler, Freiburg, for recording the high-field NMR spectra and Mr. J. A. Döhle for the cyclovoltammograms.
Dedicated to Professor Hans-Jürgen Bestmann on the occasion of his 60th birthday
Graphical Abstract
References
- 1Review: T. Shono, Tetrahedron 40 (1984) 811.
- 2 See, for example, Rote Liste: Verzeichnis von Fertigazneimitteln der Mitglieder des Bundesverbands der Pharmazeutischen Industrie, Bundesverband der Pharmazeutischen Industrie, Aulendorf 1984.
- 3See, for example, B. Lenarcik, M. Wisniewski, M. Gabryszewski, Pol. J. Chem. 54 (1980) 1869; B. F. Abdullin, G. G. Sadikov, M. G. Lutfullina, T. A. Malikova, M. A. Porai-Koshits, Kristallografiya 25 (1980) 397; Chem. Abstr. 93 (1980) 35323e; N. S. Rukk, G. P. Kuznetsova, A. P. Belousova, L. Y. Alikberova, B. D. Stepin, Zh. Neorg. Khim. 28 (1983) 1623; Chem. Abstr. 99 (1983) 44177m, and references cited therein; nitrite (also bromate): K. G. Weiss, D. F. Boltz, Anal. Chim. Acta 55 (1971) 77; M. Qureshi, S. Z. Qureshi, N. Zehra, Mikrochim. Acta. 1970, 831.
- 4See, for example, G. C. Khan, A. R. Boobis, S. Murray, D. S. Davies, Xenobiotica 12 (1982) 509; J. A. Holme, A. Eek-Hansen, K. F. Jervell, Acta Pharmacol. Toxicol. 50 (1982) 272, and references cited therein.
- 5 Compound 4 afforded satisfactory elemental analysis. 4: 1H-NMR (80 MHz, CDCl3):δ = 7.6–7.15 (m,5H), 5.48 (q, 1H,J = 1 Hz), 4.81 (s, 2H), 3.23 (s, 3H), 2.31 (d, 3H, J = 1 Hz; for 1, the same allylic couplingis observed); 13C-NMR (20.1 MHz, CDCl3):δ = 166.6, 155.6, 135.1, 129.0 (2C), 126.7, 124.3 (2C), 100.7, 76.6, 57.7, 12.3; UV (CH3OH): λmax(ϵ) = 240 (10 150), 262 (9600) nm; IR (KBr): V = 1660 cm−1 (CO); MS (70 eV): m/z 218 (14%,M⊙⊕), 187 (6), 186 (6), 45 (100).
- 6
For example, exclusively reactions at the unsubstituted ring C atom and at the C-CH3 of 1 occur upon bromination with N-bromosuccinimide:
H. De. Greaf,
J. Ledrut,
G. Combes,
Bull. Soc. Chim. Belg.
61 (1952) 331;
10.1002/bscb.19520610702 Google Scholarmore extensive oxidations with CeIV or K3Fe(CN)6 or H2O2: H. Tomankova, J. Zyka, Microchem. J. 19 (1974) 86; Microchem. J. 20 (1975) 132, 367; S. Saxena, J. D. Pandey, Z. Anal. Chem. 262 (1972) 368.
- 7 A. R. Boobis, M. J. Brodie, G. C. Kahn, E. L. Toverud, I. A. Blair, Br. J. Clin. Pharmacol. 12 (1981) 771; M. Danhof, A. van Zuilen, J. K. Boeijinga, D. D. Breimer, Eur. J. Clin. Pharmacol. 21 (1982) 433; H. Uchino, T. Inaba, W. Kalow, Xenobiotica 13 (1983) 155; hydroxylation of the phenyl group of 1: J. Boettcher, H. Baeszmann, R. Schueppel, Dev. Bio chem. 23 (1982) 329; Dev. Bio chem. 13 (1980) 81.
- 8 Under the preparative conditions (2% KOH in CH3OH,Pt), current-voltage curves versus the Ag/AgCl/sat. KCl electrode gave potentials of 1.43 V for the basic solvent (equally in the presence of 0.10M4) and 1.30 for a 0.10 M solution of 1 for currents of 2A (ca. 40 mA/cm2).
- 9 Tautomerism of 5: A. R. Katrizky, F. W. Maine, Tetrahedron 20 (1964) 299.
- 10 6:1H-NMR (250 MHz, CDCl3, 22°C): δ = 7.8–7.65 (m, 4H), 7.4–7.15 (m, 6H), 3.90 (≥2H, very br. s, Δv1/2 ca. 180 Hz), 3.27 (2H, br. s, Δv1/2 = 5 Hz), 2.32 (6H, br. s, Δv1/2 = 5.5 Hz); UV (CH3OH): λmax = 242, 260 nm (sh); IR (KBr): V = 3340 (OH), 1620 (sh), 1610 (sh), 1600, 1560, 1550, 1505 cm−1 (C = N, C = C); MS(70 eV): m/z 360 (1%, M⊙⊕), 358(3), 187(13), 186(67), 185(44), 174(64), 157(12), 105(32), 91(53), 77(100); for compound 6, the diketo form (4,4′-methylenebis (5-methyl-2-Phenyl-2H, 4H-Pyrazol-3-one)) has been postulated up to now: U. Wrzeciono, Justus Liebigs Ann. Chem. 1975, 2293, and references cited therein.
- 11
L. Knorr,
Justus Liebigs Ann. Chem.
238 (1887) 137;
10.1002/jlac.18872380107 Google ScholarJustus Liebigs Ann. Chem. 293 (1896) 1; 1H-NMR(CDCl3): 7: δ = 7.5–7.2 (m, 5H), 5.36 (q, 1H, J = 1 Hz), 3.52 (q, 2H, J = 7Hz), 2.20 (d 3H, J = 1Hz), 0.85 (t, 3H, J = 7Hz); 8:δ = 7.5–7.2 (m, 5H), 2.93 (s, 3H), 2.14 (q, 3H, J = 1 Hz), 1.84(q, 3H, J = 1 Hz); 9:δ = 7.5–7.2 (m, 5H),4.78 (s, 2H), 3.23 (s, 3H), 2.25 (q, 3H), (J = 1 Hz), 1.87 (q, 3H, J = 1 Hz); yield of 9 determined by 1H-NMR spectroscopy for 53% conversion of 8: 96%.10.1002/jlac.18962930102 Google Scholar
- 12 A striking parallel is found in the electrochemical methoxylation of amides. Thus, in the review [1], no methoxylations of N-ethylamides are described; the numerous examples are for N-methylamides, cyclic acylated amines, and lactams.
- 13 H. Sayo, M. Masui, Chem. Pharm. Bull. 24 (1976) 2137; J. Chem. Soc. Perkin Trans. 2 1973, 1640: By cyclovoltammetry (6 V/s, CH3CN, 0.1 M NaClO4 vs. SCE) anodic potentials of 1.22 and 1.65 V for 1 were measured; in contrast, the anodic potentials of 4-dimethylamino-and 4-diethylaminoantipyrine are, as expected, lower (0.34, 0.85, 1.32 and 0.39, 0.88, 1.18, 1.38 V, respectively) and it was possible to detect spectroscopically the blue-violet radical cations, from which (with the involvement, this time, of the C- methyl group and not the N-methyl group), the decomposition products acetaldehyde and diethylamine were formed in low yield.