Enantio- and anti-Diastereoselective Aldol Additions of Acetates and Propionates via O-Silyl Ketene Acetals†
This work was supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie. We thank F. Lippert for carrying out some of the experiments, Prof. R. Baker, Southampton, for data on the optical rotation of 19, and Prof. B. Ganem, Cornell University, for a sample of ent-20. For definition of the descriptors syn and anti see [4a].



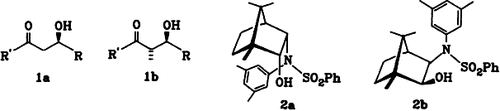
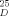 −9.4 (c = 0.6 CHCl3); 19 (cf.[4c]):[α]D −7.5 (c = 1.8, CH2Cl2); 20 from 16:[α]
−9.4 (c = 0.6 CHCl3); 19 (cf.[4c]):[α]D −7.5 (c = 1.8, CH2Cl2); 20 from 16:[α]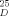 + 11.3 (c = 0.6, CHCl3); ent- 20 (vgl.[11]):[α]
+ 11.3 (c = 0.6, CHCl3); ent- 20 (vgl.[11]):[α]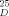 −10.3 (c = 0.2, CHCl3).
−10.3 (c = 0.2, CHCl3).

