Journal list menu
Export Citations
Download PDFs
Cover Pictures
Cover Picture: Umpolung by N-Heterocyclic Carbenes: Generation and Reactivity of the Elusive 2,2-Diamino Enols (Breslow Intermediates) (Angew. Chem. Int. Ed. 49/2012)
- Page: 12135
- First Published: 09 November 2012

Breslow intermediates of N-heterocyclic carbene (NHC) catalyzed Umpolung reactions have been generated by combining saturated NHCs with aldehydes. In their Communication on page 12370 ff., A. Berkessel et al. report the observation of these pivotal but hitherto elusive intermediates. Indeed, much water has flown down the Rhine since the first postulation of amino enols as the “umpoled” form of aldehydes in 1958 until their characterization reported herein! (Graphics & photo: Adrian von der Höh.)
Inside Cover: Preferential Formation of Cyclic Trimers by Palladium-Catalyzed Oxidative Coupling Reactions of 2,18-Diethynylporphyrins (Angew. Chem. Int. Ed. 49/2012)
- Page: 12136
- First Published: 23 November 2012

Cyclic porphyrin oligomers are difficult to obtain efficiently. In their Communication on page 12357 ff., A. Osuka, H. Yorimitsu, and S. Tokuji show Pd-catalyzed trimerization of 2,18-diethynylporphyrins. Hexaporphyrins with meso–meso directly linked porphyrins can be used to model a light-harvesting antenna. The crystal structure of the trimer resembles the family crest of Mitsuba-aoi (three leaves of Hollyhock), which is the famous crest of the Tokugawa clan.
Inside Back Cover: Discovery of Small-Molecule Inhibitors of the TLR1/TLR2 Complex (Angew. Chem. Int. Ed. 49/2012)
- Page: 12375
- First Published: 14 November 2012

Toll-like receptors (TLRs) play pivotal roles in innate immunity. Two key members of the TLR protein family, TLR1 (orange) and TLR2 (magenta), form a heterodimeric complex that recognizes pathogen-associated molecule patterns such as Pam3CSK4 (green) and activates inflammation response. On page 12246 ff., H. Yin and co-workers describe the new inhibitor CU-CPT22 (white), which displaces bound Pam3CSK4 from the TLR1–TLR2 complex, thus providing a valuable probe for these important signaling receptors.
Back Cover: A Supramolecular System for the Electrochemically Controlled Release of Cells (Angew. Chem. Int. Ed. 49/2012)
- Page: 12376
- First Published: 22 November 2012

Electrochemically activated cell release by using redox-active supramolecular complexes assembled on microelectrodes is described by P. Jonkheijm et al. in their Communication on page 12233 ff. Host molecule cucurbit[8]uril links surface-bound viologen to solution-exposed RGD peptides to form a cell-adhesive surface. Electrochemical reduction results in dissociation of the supramolecular complex, release of the RGD peptides, and detachment of cells from the surfaces.
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 49/2012
- Pages: 12139-12152
- First Published: 28 November 2012
Flashback
50 Years Ago...
- Page: 12148
- First Published: 28 November 2012
Angewandte Chemie International Edition was first published in 1962, the mother journal first in 1888. In this monthly flashback, we feature some of the articles that appeared 50 years ago. This look back can open our eyes, stimulate discussion, or even raise a smile.
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 49/2012
- Pages: 12154-12157
- First Published: 28 November 2012
Author Profile
News
Société Chimique de France 2012 Prize Winners
- Pages: 12162-12163
- First Published: 09 November 2012
Book Review
Sand and Silicon. Science that Changed the World. By Denis McWhan.
- Pages: 12164-12165
- First Published: 28 November 2012
Highlights
Dehydrative Alkylation
Ruthenium-Catalyzed ortho-Alkylation of Phenols with Alcohols by Dehydrative Coupling†
- Pages: 12166-12168
- First Published: 25 October 2012
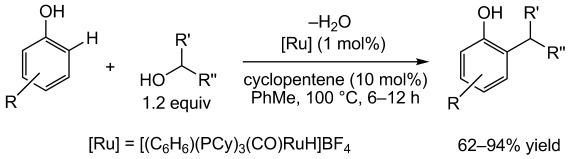
Only water: Phenols have been shown to undergo a ruthenium-catalyzed ortho-alkylation reaction (see scheme; Cy=cyclohexyl). Unactivated alcohols are used as the alkylating agents and water is the only by-product that is generated, making this transformation more atom-economical and greener than traditional methods.
1-Azidoalkenes and 1-Azidoalkynes
Vinyl and Alkynyl Azides: Well-Known Intermediates in the Focus of Modern Synthetic Methods
- Pages: 12169-12171
- First Published: 24 October 2012
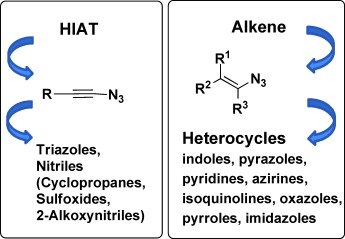
Getting real: Although 1-azidoalkenes are known as important intermediates for the formation of many nitrogen heterocycles, for a long time the existence of the corresponding 1-azidoalkynes could not be proven unequivocally. The recent synthesis and full characterization of 1-azidoethyne have provided incontrovertible verification of this substance class.
Nobel Prize for Chemistry 2012
The Seven Pillars of Molecular Pharmacology: GPCR Research Honored with Nobel Prize for Chemistry
- Pages: 12172-12175
- First Published: 05 November 2012
Review
Life in a Bubble
Droplet Microfluidics—A Tool for Single-Cell Analysis
- Pages: 12176-12192
- First Published: 23 November 2012

A one-off: Single-cell analysis is one of the most interesting applications for droplet microfluidics. Droplets provide robust compartments on the size scale of a single cell, and their ability to encapsulate and rapidly manipulate cells along with their immediate environment in monodisperse compartments allows the possibility of automation. This Review focuses on single-cell analyses and applications in drug screening and genetic and enzyme analysis.
Communications
Supramolecular Chemistry
Pentadecker Supramolecules with a Lithium Alkoxo Nanobelt Sandwiched between Two Highly Charged Buckybowl Surfaces†
- Pages: 12194-12198
- First Published: 22 October 2012
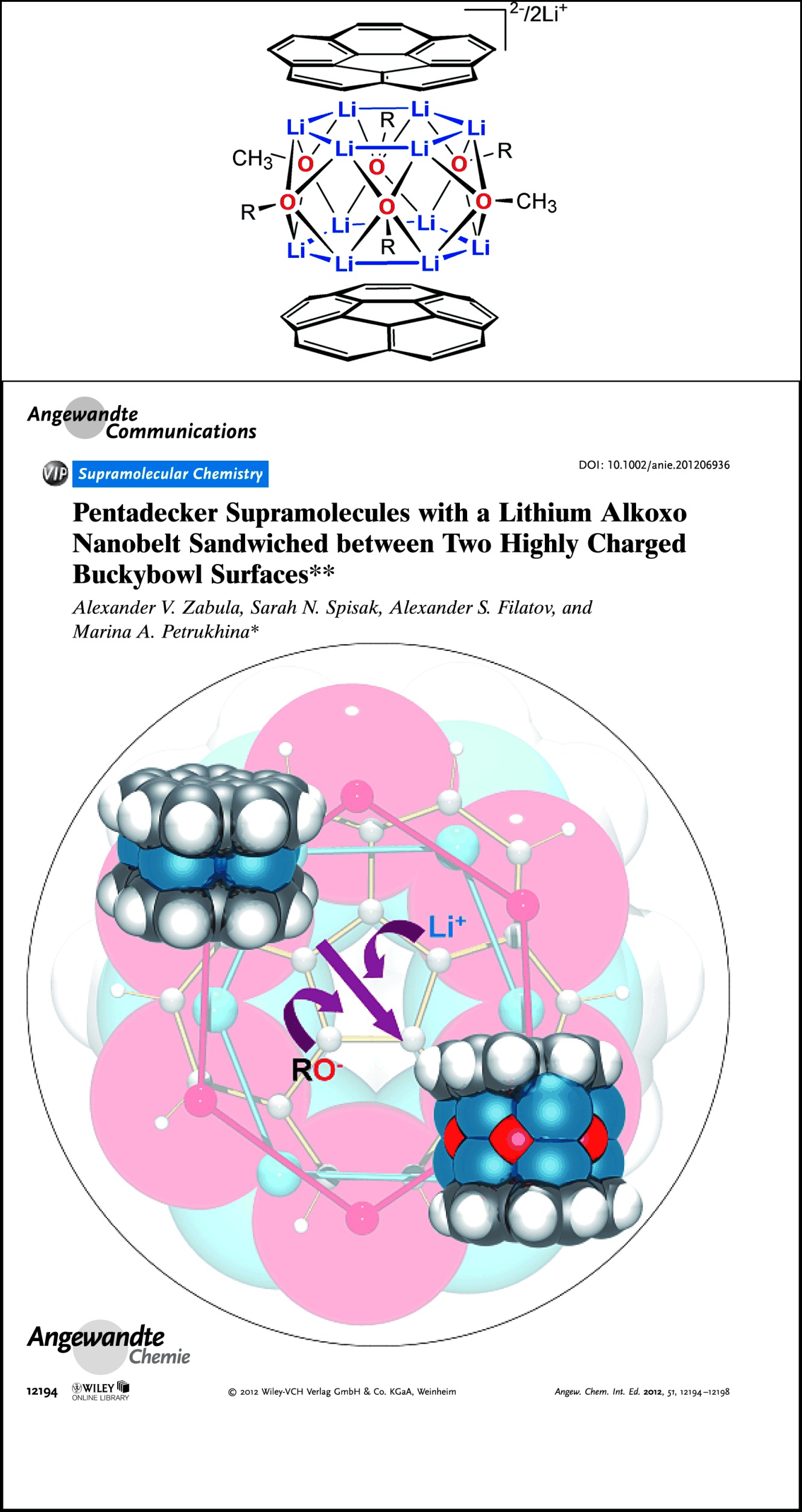
Stack it up: Reduction of corannulene with an excess of lithium metal in dimethoxyethane (DME) results in the formation of remarkable multilayered supramolecular aggregates (see picture) having lithium alkoxo nanobelts encapsulated between two tetrareduced corannulene bowls, [C20H104−/Li6(OR)6Li6/C20H104−]2−. The alkoxo groups (R=CH3O or/and CH3OCH2CH2O) are products of the Li-induced DME cleavage.
Metal Ion Clusters
Triple-Decker Au3–Ag–Au3–Ag–Au3 Ion Cluster Enclosed in a Self-Assembled Cage†
- Pages: 12199-12201
- First Published: 26 October 2012
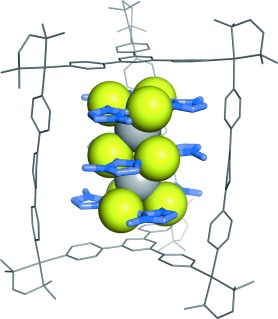
Silver in a gold mine: A triple-decker AuI–AgI ion cluster was synthesized by alternate alignment of cyclic trinuclear AuI complexes and AgI ions within a self-assembled cage. The box-shaped cavity of the cage is suitable not only for limiting the cluster number, but also for the stabilization of weakly associated metal ions, which cannot exist without the help of the cage.
Nanostructures
A General Strategy for the Synthesis of Carbon Nanofibers from Solid Carbon Materials†
- Pages: 12202-12205
- First Published: 29 October 2012
Graphene
A Boron-Containing PAH as a Substructure of Boron-Doped Graphene†
- Pages: 12206-12210
- First Published: 18 October 2012
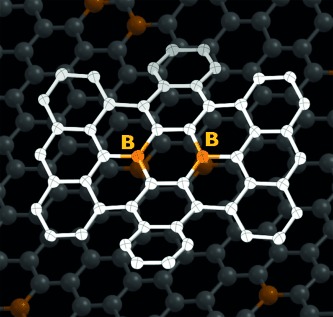
BBC news: Two boron atoms have been incorporated into a carbon nanosheet by a bottom-up synthesis to give a “B-doped nanographene” (see picture) with a defined structure. The experimental and theoretical analyses revealed the important role of the two boron atoms in producing its characteristic properties, such as broad absorption bands covering the visible region and reversible redox processes.
SiC Activation
Cascade Activation of SiH, CH, and SiC Bonds at a Rhodium β-Diiminate Complex†
- Pages: 12211-12214
- First Published: 04 November 2012
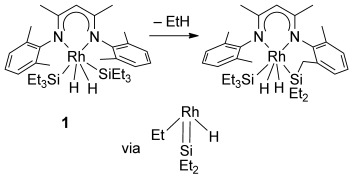
The coupling of HSiEt3 and related silanes to β-diiminate ligands is achieved through a series of SiH, CH, and SiC bond-breaking and bond-forming reactions. Complex 1 (see scheme) loses an Et(Si) group as ethane and couples the remaining SiEt2H fragment to a benzylic methyl group of the ligand skeleton. According to DFT calculations, the reaction involves silylene intermediates.
Carbon Nanodots
A Biocompatible Fluorescent Ink Based on Water-Soluble Luminescent Carbon Nanodots†
- Pages: 12215-12218
- First Published: 29 October 2012

C-dots on hand: Luminescent carbon nanodots were synthesized and were shown to be biocompatible, have low toxicity, and distinctive photoluminescence properties. These C-dots are inexpensive to synthesize and could potentially be used for versatile applications, such as anticounterfeiting, information encryption, and information storage.
Heterocycle Synthesis
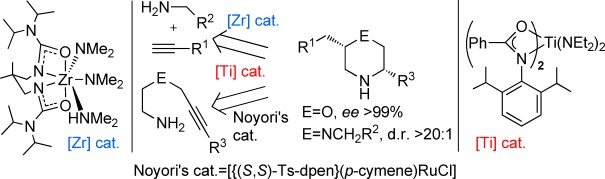
Catalytic Asymmetric Synthesis of Substituted Morpholines and Piperazines†
- Pages: 12219-12223
- First Published: 26 October 2012
Gold Nanoparticles
Visualization of Latent Fingermarks by Nanotechnology: Reversed Development on Paper—A Remedy to the Variation in Sweat Composition†
- Pages: 12224-12227
- First Published: 04 November 2012
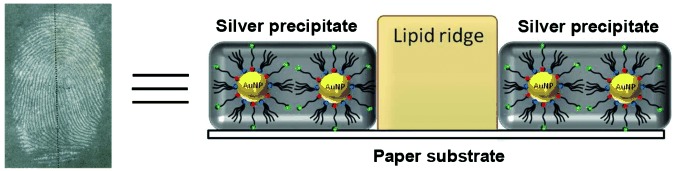
Don′t sweat it: “Negative” fingermarks are developed on paper by the application of gold nanoparticles (gold circles) that are capped by a bifunctional ligand, and then silver precipitation. In this process, paper is the substrate and the fingermarks serve as a mask. This approach may contribute to the successful recovery of latent fingermarks by law enforcement agencies.
Molecular Electronics
Building High-Throughput Molecular Junctions Using Indented Graphene Point Contacts†
- Pages: 12228-12232
- First Published: 04 November 2012
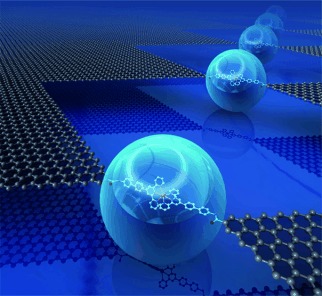
A molecular-scale gap array is introduced into a single-layer graphene sheet by a lithographic dash-line cutting process. Electrically active molecules are then covalently wired into these point contacts in high yield, thus forming stable molecular devices that for example are able to reversibly switch their conductance by chemical treatment.
Host–Guest Systems
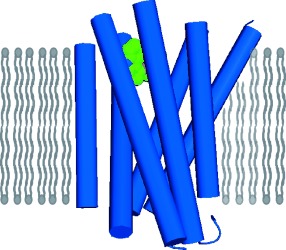
A Supramolecular System for the Electrochemically Controlled Release of Cells†
- Pages: 12233-12237
- First Published: 04 November 2012
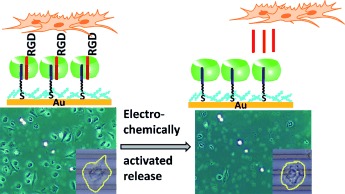
Please release me: Electrochemically activated cell release is achieved using a redox-active supramolecular complex (see picture). Host molecule CB[8] (green) links surface-bound viologen (purple) with solution-exposed RGD peptides (red). Electrochemical reduction dissociates the complex, releases the peptides, and thus releases the cells from the substrates. This supramolecular strategy is also applicable to microelectrodes.
Natural Products
A Combined Atomic Force Microscopy and Computational Approach for the Structural Elucidation of Breitfussin A and B: Highly Modified Halogenated Dipeptides from Thuiaria breitfussi†
- Pages: 12238-12241
- First Published: 26 October 2012

Turning a new leaf: The first structures isolated from Thuiaria breitfussi, the breitfussins, are presented. This structural class consists of indole–oxazole–pyrrole units. Limited quantities prevented crystallization; therefore, the structures were solved using a novel combination of AFM, computer-aided structure elucidation (CASE), and DFT calculations. Visualization by AFM determined all the connection points of the cyclic systems and the other substituents.
Enzyme Catalysis
Charge-Balanced Metal Fluoride Complexes for Protein Kinase A with Adenosine Diphosphate and Substrate Peptide SP20†
- Pages: 12242-12245
- First Published: 04 November 2012
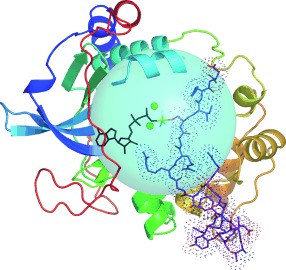
Well-balanced: 19F NMR spectroscopy defined a trifluoromagnesate complex for protein kinase A (multicolored) with adenosine diphosphate (black), the MgF3− ion (green), and the SP20 peptide substrate (purple with dots). A sphere (cyan) centered on the MgF3− ion embraces all catalytic components and much of the SP20 substrate. The content of the sphere is uncharged conforming to the charge balance hypothesis.
Drug Discovery
Discovery of Small-Molecule Inhibitors of the TLR1/TLR2 Complex†
- Pages: 12246-12249
- First Published: 11 September 2012
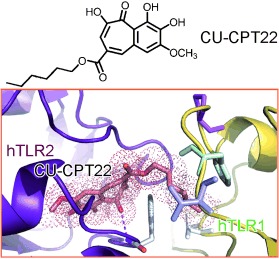
An important regulator of innate immunity, the protein complex of Toll-like receptors 1 and 2 (TLR1/TLR2) provides an attractive target for the treatment of various immune disorders. The novel compound CU-CPT22 can compete with the binding of the specific lipoprotein ligand to TLR1/TLR2 (see picture) with high inhibitory activity and specificity. Repression of downstream signaling from TNF-α and IL-1β was also observed.
Cross-Coupling
Tandem Heck/Decarboxylation/Heck Strategy: Protecting-Group-Free Synthesis of Symmetric and Unsymmetric Hydroxylated Stilbenoids†
- Pages: 12250-12253
- First Published: 04 November 2012
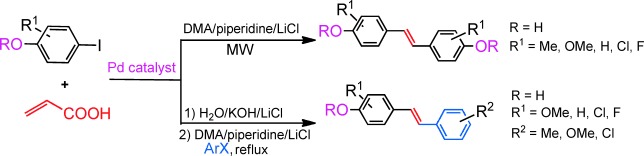
Ride the (micro)wave: The title strategy has been developed for the synthesis of various symmetric or unsymmetric hydroxylated stilbenoids utilizing 4-halophenols and acrylic acid as coupling partners (see scheme; DMA=N,N-dimethylacetamide, MW=microwave). Protecting groups are not necessary and a single palladium catalyst is used.
Sequence-Controlled Polymerization
Polymer-Chain Encoding: Synthesis of Highly Complex Monomer Sequence Patterns by Using Automated Protocols†
- Pages: 12254-12257
- First Published: 29 October 2012
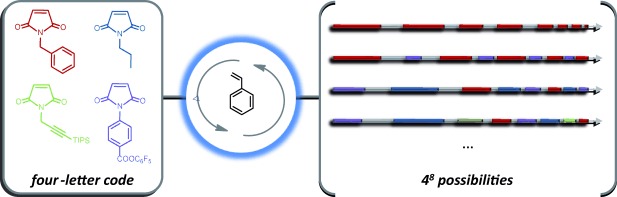
The automated sequence-controlled copolymerization of styrene and N-substituted maleimides allowed the production of unprecedented polymer microstructures (up to 65536 possible microstructural arrangements). Highly complex monomer sequence patterns were prepared using four N-substituted maleimides (see picture).
Protein Expression
Trans-Platinum/Thiazole Complex Interferes with Sp1 Zinc-Finger Protein†
- Pages: 12258-12262
- First Published: 04 November 2012
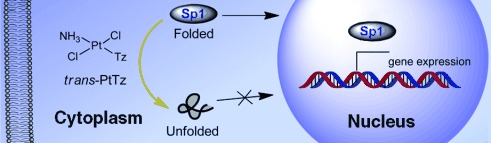
No trafficking: The antitumor-active trans-platinum/thiazole complex trans-PtTz demonstrated high reactivity to the transcription factor Sp1, which is overexpressed in tumor cells. The binding of trans-PtTz disrupts the DNA interaction with Sp1 in vitro and prevents the protein trafficking from the cytoplasm into the nucleus (see picture).
Protein Synthesis
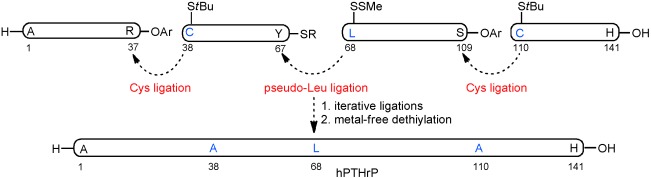
Chemistry as an Expanding Resource in Protein Science: Fully Synthetic and Fully Active Human Parathyroid Hormone-Related Protein (1–141)†
- Pages: 12263-12267
- First Published: 04 November 2012
Nanostructures
Integrated and Segregated Au/γ-Fe2O3 Binary Nanoparticle Assemblies†
- Pages: 12268-12271
- First Published: 07 November 2012
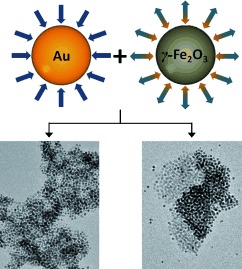
To mix or not to mix: Integrated (left) and segregated (right) assemblies were obtained upon treating functionalized γ-Fe2O3 nanoparticles (NPs) with AuNPs. Their binary nature is controlled by the capping layer of the γ-Fe2O3 NPs and the AuNP initial aggregation state. The segregated assembly formation is induced by AuNP aggregates which act as nucleation sites for growth of the γ-Fe2O3 NP domains.
Fingerprints
Visualization of Latent Fingermarks Using an Aptamer-Based Reagent
- Pages: 12272-12274
- First Published: 29 October 2012

Don′t touch! Aptamers selected against lysozyme are transformed into aptamer-based reagents, with which latent fingermarks can be developed with high selectivity and sensitivity. The design of aptamers targeting components of latent fingermarks opens up a new range of detection methods that previously have not been explored.
Organofluorine Chemistry
CF Bond Activation of Unactivated Aliphatic Fluorides: Synthesis of Fluoromethyl-3,5-diaryl-2-oxazolidinones by Desymmetrization of 2-Aryl-1,3-difluoropropan-2-ols†
- Pages: 12275-12279
- First Published: 04 November 2012
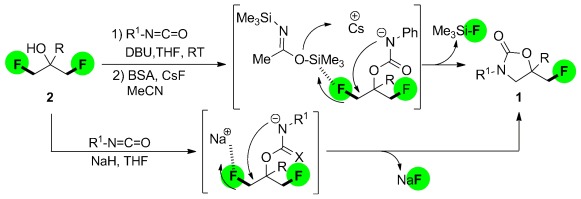
How to lose fluorine: Biologically relevant oxazolidinones 1 were synthesized through the desymmetrization of unactivated aliphatic difluorides by Si-induced catalytic CF bond-cleavage using BSA/CsF (BSA=bis(trimethylsilyl)acetamide). The direct transformation of 2 with isocyanates into 1 by cascade carbamoylation/cyclization, in which cyclization is induced by Na-assisted CF bond activation, was also achieved.
Uranium Siloxides
Siloxides as Supporting Ligands in Uranium(III)-Mediated Small-Molecule Activation†
- Pages: 12280-12284
- First Published: 04 November 2012
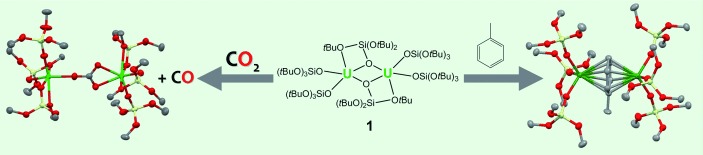
Siloxides can support U! …︁in the reduction of small molecules with uranium complexes. The treatment of [U{N(SiMe3)2}3] with HOSi(OtBu)3 (3 equiv) yielded a novel homoleptic uranium(III) siloxide complex 1, which acted as a two-electron reducing agent toward CS2 and CO2 (see scheme). Complex 1 also reduced toluene to afford a diuranium inverted-sandwich complex.
Sugar Synthesis
Easy Access to L-Mannosides and L-Galactosides by Using CH Activation of the Corresponding 6-Deoxysugars†
- Pages: 12285-12288
- First Published: 29 October 2012
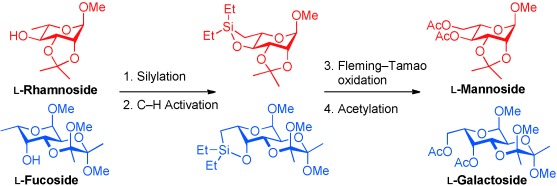
Rare sugars by CH activation: The title compounds have been prepared from the corresponding 6-deoxysugars by an intramolecular CH activation in high yields. Diethyl silane is attached to the 4-OH group followed by formation of a SiC bond; both transformations are catalyzed by iridium in a one-pot fashion. The subsequent Fleming–Tamao oxidation provided the L-sugars.
Tetrasubstituted Carbon Centers
Copper/Titanium Catalysis Forms Fully Substituted Carbon Centers from the Direct Coupling of Acyclic Ketones, Amines, and Alkynes†
- Pages: 12289-12292
- First Published: 26 October 2012
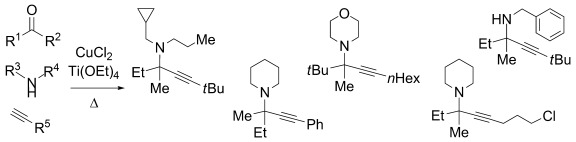
Three-component coupling, with a pair: The combination of CuII and TiIV catalyzes the first three-component coupling of unactivated ketones with a diverse range of amines and terminal alkynes. Tetrasubstituted propargylamines are formed under green, solvent-free conditions (see scheme). This dual metal system overcomes the barrier to ketimine formation and subsequent attack, opening a new path to multicomponent reactions of ketone electrophiles.
Polycyclic Hydrocarbons
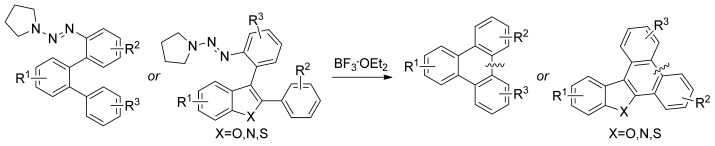
Friedel–Crafts Arylation for the Formation of C C
C Bonds: A Route to Unsymmetrical and Functionalized Polycyclic Aromatic Hydrocarbons from Aryl Triazenes†
Bonds: A Route to Unsymmetrical and Functionalized Polycyclic Aromatic Hydrocarbons from Aryl Triazenes†
- Pages: 12293-12297
- First Published: 04 November 2012
Catalytic Intermediates
A Remarkable Switch from a Diamination to a Hydrohydrazination Catalyst and Observation of an Unprecedented Catalyst Resting State†
- Pages: 12298-12302
- First Published: 04 November 2012
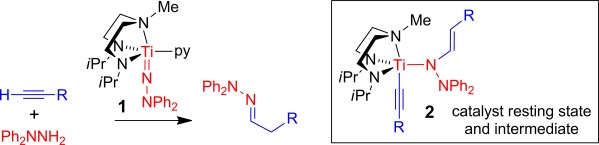
A small change with a big effect: Reaction of the titanium complex 1 with terminal alkynes gave the unusual acetylide–vinylhydrazide(1−) compounds 2 (R=4-C6H4X (X=Me, CF3, OMe), C6F5, SiMe3) as hydrohydrazination intermediates. In contrast, a complex similar to 1 with bulkier trimethylsilyl substituents in place of the isopropyl groups is known to catalyze the 1,2-diamination of alkynes with hydrazines.
Photocatalysis
Visible-Light-Induced Photocatalytic Reductive Transformations of Organohalides†
- Pages: 12303-12306
- First Published: 04 November 2012
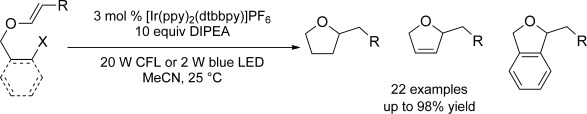
A photo opportunity: A visible-light-excited iridium catalyst delivers electrons from an amine to an organohalide. The electron transfer then induces reductive scission of the carbon–halogen bond, generating the corresponding alkyl, alkenyl, and aryl radical that can undergo cyclization and hydrodehalogenation reactions.
CH Activation
Metal-Free Oxidation/C(sp3)H Functionalization of Unactivated Alkynes Using Pyridine-N-Oxide as the External Oxidant†
- Pages: 12307-12310
- First Published: 26 October 2012
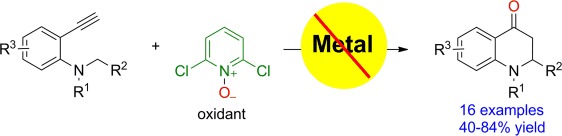
Externally yours: 2,3-Dihydroquinolin-4(1H)-ones are obtained in moderate to good yields (40–84 %, see scheme) in a metal-free oxidation/C(sp3)H functionalization of unactivated aryl alkynes. 2,6-Dichloropyridine-N-oxide is used as an external oxidant. In the reaction, a Brønsted acid, not a metal, plays a key role in the triple CC bond activation.
Heterocycles
Palladium-Catalyzed Coupling of ortho-Alkynylanilines with Terminal Alkynes Under Aerobic Conditions: Efficient Synthesis of 2,3-Disubstituted 3-Alkynylindoles†
- Pages: 12311-12315
- First Published: 04 November 2012
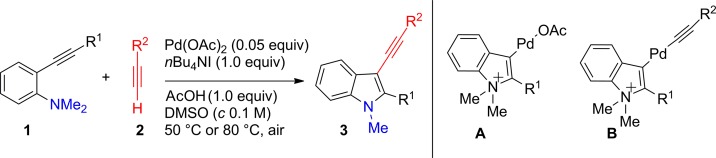
Two nucleophiles, one triple bond: Under aerobic conditions, palladium-catalyzed direct coupling of o-alkynylanilines and terminal alkynes took place smoothly to afford the 2,3-disubstituted 3-alkynylindoles 3 in good to excellent yields. The intermediate A was characterized and a retro-aminopalladation of B was observed for the first time.
Dominoreaktionen
Tandem Brønsted Acid Promoted and Nazarov Carbocyclizations of Enyne Acetals to Hydroazulenones†
- Pages: 12316-12320
- First Published: 04 November 2012
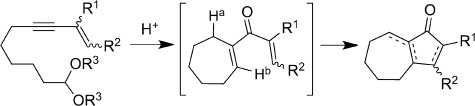
Ring the changes: Enyne acetals were easily converted into hydroazulene skeletons by a new and efficient metal-free route involving a Brønsted acid promoted carbocyclization and a subsequent stereospecific Nazarov cyclization (see scheme). The versatility of this transformation also allowed assembly of interesting heteroaromatic tricyclic systems.
Polycyclic Aromatic Hydrocarbons
Electron Acceptors Based on an All-Carbon Donor–Acceptor Copolymer†
- Pages: 12321-12324
- First Published: 29 October 2012
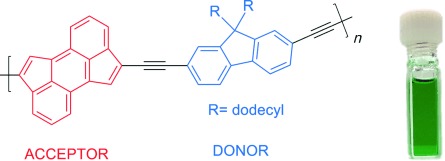
The Sonogashira cross-coupling polymerization of a dibrominated cyclopenta[hi]aceanthrylene and a diethynylfluorene derivative produced a donor–acceptor copolymer composed solely of cyclopenta-fused polycyclic aromatic hydrocarbons. The resulting polymer displays low band gaps (<1.5 eV), dual absorption bands, and electron-accepting behavior as demonstrated through fluorescence quenching of poly(3-hexylthiophene).
Micelles
Nanosized Poly(ethylene glycol) Domains within Reverse Micelles Formed in CO2†
- Pages: 12325-12329
- First Published: 26 October 2012
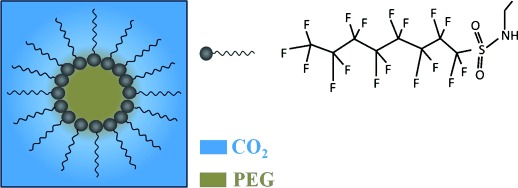
Tiny reactors: By using a surfactant (see picture), domains of poly(ethylene glycol) (PEG) at the nanometer scale are dispersed within reverse micelles formed in supercritical CO2. The size and properties of the PEG domains are tuneable by changing the PEG content. Furthermore, the PEG domains have been used as nanoreactors to synthesize highly dispersed gold nanocrystals.
Heterocycles
N-Heterocyclic-Carbene-Catalyzed Asymmetric Oxidative Hetero-Diels–Alder Reactions with Simple Aliphatic Aldehydes†
- Pages: 12330-12333
- First Published: 04 November 2012
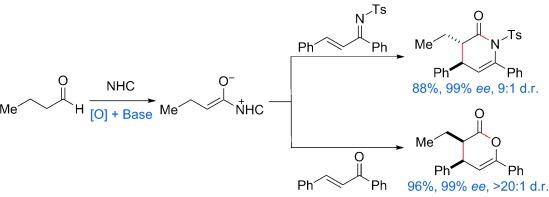
An efficient enantioselective approach to form trans lactams and cis lactones in up to 98 % yield with greater than 99 % ee, and greater than 20:1 d.r. using simple aliphatic aldehydes has been developed. The process involves a new pathway to generate enolate intermediates from aliphatic aldehydes by oxidation and deprotonation. NHC=N-heterocyclic carbene, Ts=4-toluenesulfonyl.
CC Activation
Direct Exchange of a Ketone Methyl or Aryl Group to Another Aryl Group through CC Bond Activation Assisted by Rhodium Chelation†
- Pages: 12334-12338
- First Published: 04 November 2012
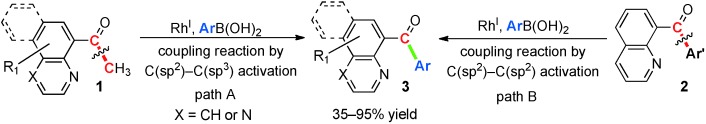
Swapped: Commercially available quinolinone derivatives (1 or 2, see scheme) were reacted with arylboronic acids in the presence of a RhI complex to give aryl(quinolin-8-yl)methanone products 3 in medium to good yields. A mechanism that involves the in situ oxidation of RhI to RhIII by O2 in the presence of CuI was proposed.
Asymmetric Catalysis
Hydrogen-Bond-Mediated Supramolecular Iminium Ion Catalysis†
- Pages: 12339-12342
- First Published: 04 November 2012
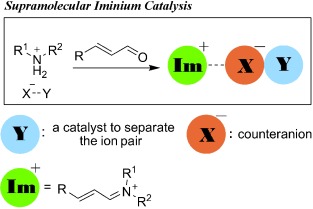
Mod squad: Multiple small molecules appear to spontaneously self-assemble to form supramolecular amine catalysts that have high reactivity, good efficiency, and enhanced turnover numbers. These modular organocatalysts should be applicable to many iminium as well as hydrogen-bond catalytic reactions and provide new insights into asymmetric catalysis.
CH Activation
Rhodium(III)-Catalyzed Oxidative CH Coupling of N-Methoxybenzamides with Aryl Boronic Acids: One-Pot Synthesis of Phenanthridinones†
- Pages: 12343-12347
- First Published: 26 October 2012
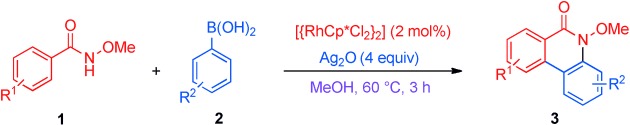
General solution: An efficient rhodium-catalyzed dual CH bond activation and cyclization of N-methoxybenzamides 1 with aryl boronic acids 2 (see scheme; Cp*=Me5C5) provides a straightforward and general approach to the phenanthridinone structure, which occurs widely in natural products and drugs. Highly regioselective CC and CN bond formation under mild conditions afforded a wide range of substituted phenanthridinones 3.
Diverse Reactivity in a Rhodium(III)-Catalyzed Oxidative Coupling of N-Allyl Arenesulfonamides with Alkynes†
- Pages: 12348-12352
- First Published: 29 October 2012
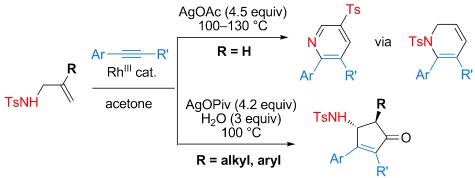
Olefinic CH activation of N-allyl sulfonamides in the presence of [{RhCp*Cl2}2] (Cp*=Me5C5) enabled their oxidative coupling with alkynes to generate 1,2-dihydropyridines, pyridines, and cyclopentenones (see scheme; Ts=p-toluenesulfonyl). The type of highly substituted product formed was controlled by the substitution of the allyl group and the reaction conditions.
Asymmetric Synthesis
Rhodium-Catalyzed Enantioselective Nucleophilic Fluorination: Ring Opening of Oxabicyclic Alkenes†
- Pages: 12353-12356
- First Published: 04 November 2012
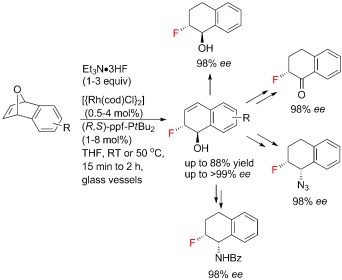
Done with 'F'lair: Enantioselective fluorination was achieved by RhI-catalyzed ring opening of oxabicyclic alkenes using Et3N⋅3 HF. The chiral fluorinated scaffolds were obtained under mild reaction conditions in standard glass vessels, and served as useful building blocks for various chiral fluorinated targets. cod=cycloocta-1,5-diene, ppf=phenylphosphinoferrocene, THF=tetrahydrofuran.
Porphyrin Macrocycles
Preferential Formation of Cyclic Trimers by Palladium-Catalyzed Oxidative Coupling Reactions of 2,18-Diethynylporphyrins†
- Pages: 12357-12361
- First Published: 09 November 2012
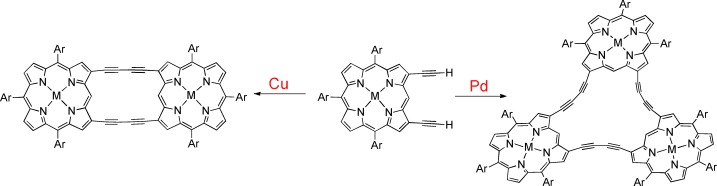
And palladium makes three: In contrast to the formation of cyclic dimers in the Cu-mediated reaction, Pd-catalyzed oxidative coupling of 2,18-diethynylporphyrins preferentially produced cyclic trimers (see scheme). A porphyrin hexamer with a doubly 1,3-butadiyne-bridged conjugated trimeric core and directly meso-appended peripheral porphyrin substituents was also synthesized.
Polymer Synthesis
Radical/Anionic SRN1-Type Polymerization for Preparation of Oligoarenes†
- Pages: 12362-12366
- First Published: 29 October 2012
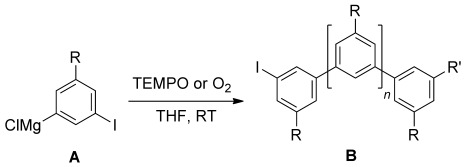
Radicals and anions: The TEMPO-mediated oxidation of magnesiated iodoarenes A provides highly regioregular poly(m-phenylenes) by strictly alternating anionic/radical cross-over chain-growth polymerization. Poly(m-phenylenes) with mean molecular weight (Mn) of up to 20 000 g mol−1 can be prepared under mild conditions by this method. The approach is a new concept for transition-metal-free polyarene synthesis. TEMPO=2,2,6,6-tetramethylpiperidine-N-oxyl radical.
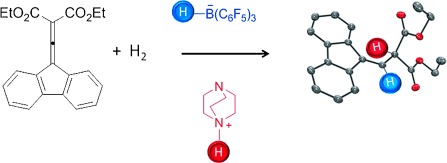
Frustrated Lewis Pairs
Metal-Free Hydrogenation of Electron-Poor Allenes and Alkenes†
- Pages: 12367-12369
- First Published: 04 November 2012
Organocatalysis
Umpolung by N-Heterocyclic Carbenes: Generation and Reactivity of the Elusive 2,2-Diamino Enols (Breslow Intermediates)†
- Pages: 12370-12374
- First Published: 18 October 2012
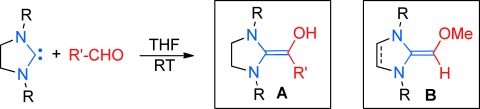
54 years later: Saturated imidazolidin-2-ylidenes react with aldehydes to smoothly produce the elusive 2,2-diamino enols A (“Breslow intermediates”, first postulated in 1958) of carbene-catalyzed umpolung reactions. The 2,2-diamino enols A react with additional aldehyde in a cross-benzoin reaction. The methylated Breslow intermediates B are accessible by deprotonation of methoxymethyl azolium salts.