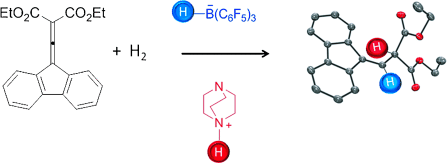
Metal-Free Hydrogenation of Electron-Poor Allenes and Alkenes†
Dr. Blanca Inés
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. David Palomas
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorSigrid Holle
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorSebastian Steinberg
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorJuan A. Nicasio
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Dr. Manuel Alcarazo
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)Search for more papers by this authorDr. Blanca Inés
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorDr. David Palomas
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorSigrid Holle
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorSebastian Steinberg
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorJuan A. Nicasio
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Search for more papers by this authorCorresponding Author
Dr. Manuel Alcarazo
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)
Max-Planck-Institut für Kohlenforschung, 45470 Mülheim an der Ruhr (Germany)Search for more papers by this authorGenerous financial support from the Fonds der Chemischen Industrie and the European Research Council (ERC Starting grant agreement no. 277963) is gratefully acknowledged. We also thank Prof. A. Fürstner for constant encouragement and the NMR spectroscopy and X-ray crystallography departments of our institute for excellent support.
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201205348_sm_miscellaneous_information.pdf4.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aS. J. Geier, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 3476–3477;
- 1bG. C. Welch, J. D. Masuda, D. W. Stephan, Inorg. Chem. 2006, 45, 478–480.
- 2M. A. Dureen, D. W. Stephan, J. Am. Chem. Soc. 2009, 131, 8396–8397.
- 3M. A. Dureen, A. Lough, T. M. Gilbert, D. W. Stephan, Chem. Commun. 2008, 4303–4305.
- 4M. A. Dureen, G. C. Welch, T. M. Gilbert, D. W. Stephan, Inorg. Chem. 2009, 48, 9910–9917.
- 5
- 5aJ. S. J. McCahill, G. C. Welch, D. W. Stephan, Angew. Chem. 2007, 119, 5056–5059;
10.1002/ange.200701215 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4968–4971;
- 5bM. Ullrich, K. S. H. Seto, A. J. Lough, D. W. Stephan, Chem. Commun. 2009, 2335–2337;
- 5cC. M. Mömming, G. Kehr, B. Wibbeling, R. Fröhlich, B. Schirmer, S. Grimme, G. Erker, Angew. Chem. 2010, 122, 2464–2467;
10.1002/ange.200906697 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2414–2417.
- 6
- 6aD. J. Parks, W. E. Piers, J. Am. Chem. Soc. 1996, 118, 9440–9441;
- 6bD. J. Parks, J. M. Blackwell, W. E. Piers, J. Org. Chem. 2000, 65, 3090–3098;
- 6cM. Alcarazo, C. Gomez, S. Holle, R. Goddard, Angew. Chem. 2010, 122, 5924–5927; Angew. Chem. Int. Ed. 2010, 49, 5788–5791;
- 6dD. Chen, V. Leich, F. Pang, J. Klankermayer, Chem. Eur. J. 2012, 18, 5184–5187.
- 7For theoretical mechanistic studies, see:
- 7aT. A. Rokob, A. Hamza, A. Stirling, T. Soós, I. Pápai, Angew. Chem. 2008, 120, 2469–2472;
10.1002/ange.200705586 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 2435–2438;
- 7bT. A. Rokob, A. Hamza, I. Pápai, J. Am. Chem. Soc. 2009, 131, 10701–10710;
- 7cS. Grimme, H. Kruse, L. Goerigk, G. Erker, Angew. Chem. 2010, 122, 1444–1447; Angew. Chem. Int. Ed. 2010, 49, 1402–1405;
- 7dfor the splitting of dihydrogen using a single carbon center, see: G. D. Frey, V. Lavallo, B. Donnadieu, W. W. Schoeller, G. Bertrand, Science 2007, 316, 439–441.
- 8
- 8aP. A. Chase, G. C. Welch, T. Jurca, D. W. Stephan, Angew. Chem. 2007, 119, 8196–8199;
10.1002/ange.200702908 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8050–8053;
- 8bP. A. Chase, T. Jurca, D. W. Stephan, Chem. Commun. 2008, 1701–1703;
- 8cC. F. Jiang, O. Blacque, H. Berke, Chem. Commun. 2009, 5518–5520;
- 8dG. Erős, H. Mehdi, I. Pápai, T. A. Rokob, P. Király, G. Tárkányi, T. Soós, Angew. Chem. 2010, 122, 6709–6713;
10.1002/ange.201001518 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 6559–6563.
- 9P. Spies, S. Schwendemann, S. Lange, G. Kehr, R. Fröhlich, G. Erker, Angew. Chem. 2008, 120, 7654–7657;
10.1002/ange.200801432 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 7543–7546.
- 10S. J. Geier, P. A. Chase, D. W. Stephan, Chem. Commun. 2010, 46, 4884–4886.
- 11H. D. Wang, R. Fröhlich, G. Kehr, G. Erker, Chem. Commun. 2008, 5966–5968.
- 12
- 12aJ. W. Yang, M. T. Hechavarria Fonseca, B. List, Angew. Chem. 2004, 116, 6829–6832; Angew. Chem. Int. Ed. 2004, 43, 6660–6662;
- 12bJ. B. Tuttle, S. G. Ouellet, D. W. C. MacMillan, J. Am. Chem. Soc. 2006, 128, 12662–12663;
- 12cM. Rueping, A. R. Antonchick, T. Theissmann, Angew. Chem. 2006, 118, 3765–3768;
10.1002/ange.200600191 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 3683–3686.
- 13Asymmetric versions of some of these transformations have been also recently developed; see:
- 13aD. Chen, J. Klankermayer, Chem. Commun. 2008, 2130–2131;
- 13bD. Chen, Y. T. Wang, J. Klankermayer, Angew. Chem. 2010, 122, 9665–9668; Angew. Chem. Int. Ed. 2010, 49, 9475–9478.
- 14Only a couple of single examples have been described, namely the reductions of carvone (see Ref. [8d]) and an ynone:
- 14aB. H. Xu, G. Kehr, R. Fröhlich, B. Wibbeling, B. Schirmer, S. Grimme, G. Erker, Angew. Chem. 2011, 123, 7321–7324; Angew. Chem. Int. Ed. 2011, 50, 7183–7186. During the review process of this manuscript, new examples have been described:
- 14bJ. S. Reddy, B. H. Xu, T. Mahdy, R. Fröhlich, G. Kehr, D. W. Stephan, G. Erker, Organometallics 2012, 31, 5638–5649.
- 15The ability of PhNMe2 in combination to B(C6F5)3 to form FLPs that are able to activate H2 has been recently reported: T. Voss, T. Mahdi, E. Otten, R. Fröhlich, G. Kehr, D. W. Stephan, G. Erker, Organometallics 2012, 31, 2367–2378. The reported acidity of the diaryl ammonium cation [Ph2NH2].+(pKa=0.78 in H2O) suggest that [Ph2MeNH]+ should be able to protonate allene 1.
- 16For the interaction of alkenes with boranes, see: X. Zhao, D. W. Stephan, J. Am. Chem. Soc. 2011, 133, 12448–12450.
- 17For the synthesis of 6, see: A. C. Dyker, V. Lavallo, B. Donnadieu, G. Bertrand, Angew. Chem. 2008, 120, 3250–3253;
10.1002/ange.200705620 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 3206–3209.
- 18CCDC 890483 (5), 890379 (7), 890482 (9), 890381 (33), and 890380 (34) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 19
- 19aC. M. Conifer, D. J. Law, G. J. Sunley, A. J. P. White, G. J. P. Britovsek, Organometallics 2011, 30, 4060–4066;
- 19bD. J. Parks, W. E. Piers, M. Parvez, R. Atencio, M. J. Zaworotko, Organometallics 1998, 17, 1369–1377.
- 20For a comprehensive discussion regarding thermal rearrangements of allenes, see: J. E. Baldwin, R. H. Fleming, Fortschr. Chem. Forsch. 1970, 15, 281–310.
- 21D. W. Stephan, S. Greenberg, T. W. Graham, P. Chase, J. J. Hastie, S. J. Geier, J. M. Farrel, C. C. Brown, Z. M. Heiden, G. C. Welch, M. Ullrich, Inorg. Chem. 2011, 50, 12338–12348, and references therein.
- 22The synthesis of this compound is described in the Supporting Information.