Vinyl and Alkynyl Azides: Well-Known Intermediates in the Focus of Modern Synthetic Methods
Corresponding Author
Dr. Nicole Jung
IOC-ComPlat and ITG; KIT-Campus North, Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen (Germany)
Nicole Jung, IOC-ComPlat and ITG; KIT-Campus North, Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen (Germany)
Stefan Bräse, Institute of Organic Chemistry (IOC), KIT-Campus South and Institute of Toxicology and Genetics (ITG), KIT-Campus North, Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Bräse
Institute of Organic Chemistry (IOC), KIT-Campus South and Institute of Toxicology and Genetics (ITG), KIT-Campus North, Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany)
Nicole Jung, IOC-ComPlat and ITG; KIT-Campus North, Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen (Germany)
Stefan Bräse, Institute of Organic Chemistry (IOC), KIT-Campus South and Institute of Toxicology and Genetics (ITG), KIT-Campus North, Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorCorresponding Author
Dr. Nicole Jung
IOC-ComPlat and ITG; KIT-Campus North, Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen (Germany)
Nicole Jung, IOC-ComPlat and ITG; KIT-Campus North, Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen (Germany)
Stefan Bräse, Institute of Organic Chemistry (IOC), KIT-Campus South and Institute of Toxicology and Genetics (ITG), KIT-Campus North, Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorCorresponding Author
Prof. Dr. Stefan Bräse
Institute of Organic Chemistry (IOC), KIT-Campus South and Institute of Toxicology and Genetics (ITG), KIT-Campus North, Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany)
Nicole Jung, IOC-ComPlat and ITG; KIT-Campus North, Hermann-von-Helmholtz Platz 1, 76344 Eggenstein-Leopoldshafen (Germany)
Stefan Bräse, Institute of Organic Chemistry (IOC), KIT-Campus South and Institute of Toxicology and Genetics (ITG), KIT-Campus North, Fritz-Haber-Weg 6, 76131 Karlsruhe (Germany)
Search for more papers by this authorGraphical Abstract
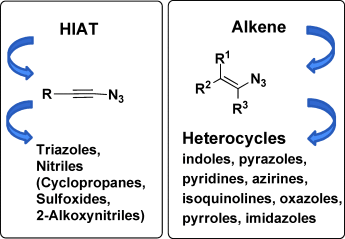
Getting real: Although 1-azidoalkenes are known as important intermediates for the formation of many nitrogen heterocycles, for a long time the existence of the corresponding 1-azidoalkynes could not be proven unequivocally. The recent synthesis and full characterization of 1-azidoethyne have provided incontrovertible verification of this substance class.
References
- 1G. L’Abbé, Angew. Chem. 1975, 87, 831–838; Angew. Chem. Int. Ed. Engl. 1975, 14, 775–782.
- 2
- 2aF. Palacios, A. M. Ocjoa de Retana, E. Martínez de Marigorta, J. M. de Los Santos, Eur. J. Org. Chem. 2001, 2401–2414;
- 2bA. G. O’Brien, F. Lévesque, P. H. Seeberger, Chem. Commun. 2011, 47, 2688–2690;
- 2cH. Zou, H. Zhu, J. Shao, J. Wu, W. Chen, M. A. Giulianotti, Y. Yu, Tetrahedron 2011, 67, 4887–4891;
- 2dY. F. Wang, K. K. Toh, E. P. J. Ng, S. Chiba, J. Am. Chem. Soc. 2011, 133, 6411–6421;
- 2eS. Sato, H. Kato, M. Ohta, Bull. Chem. Soc. Jpn. 1967, 40, 2938–2942;
- 2fE. P. J. Ng, Y.-F. Wang, B. W.-Q. Hui, G. Lapointe, S. Chiba, Tetrahedron 2011, 67, 7728–7737;
- 2gB. Hu, Z. Wang, N. Ai, J. Zheng, X.-H. Liu, S. Shan, Z. Wang, Org. Lett. 2011, 13, 6362–6365.
- 3S. Chiba, Synlett 2012, 21–44.
- 4Y. F. Wang, K. K. Toh, J.-Y. Lee, S. Chiba, Angew. Chem. 2011, 123, 6049–6053; Angew. Chem. Int. Ed. 2011, 50, 5927–5931.
- 5B. J. Stokes, H. Dong, B. E. Leslie, A. L. Pumphrey, T. G. Driver, J. Am. Chem. Soc. 2007, 129, 7500–7501.
- 6
- 6aA. Hassner, F. W. Fowler, J. Org. Chem. 1968, 33, 2686–2691;
- 6b“The Chemistry of Vinyl, Allenyl, and Ethynyl Azides”: K. Banert in Organic Azides: Syntheses and Applications (Eds.: ), Wiley, Chichester, UK, 2010;
- 6cS. Bräse, C. Gil, K. Knepper, V. Zimmermann, Angew. Chem. 2005, 117, 5320–5374;
10.1002/ange.200400657 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 5188–5240.
- 7L. Kupracz, J. Hartwig, J. Wegner, S. Ceylan, A. Kirschning, Beilstein J. Org. Chem. 2011, 7, 1441–1448.
- 8W. Zhu, D. Ma, Chem. Commun. 2004, 888–889.
- 9
- 9aV. Nair, G. G. Tesmol, Tetrahedron Lett. 2000, 41, 3199–3201;
- 9bV. N. Telvekar, B. S. Takale, H. M. Bachhav, Tetrahedron Lett. 2009, 50, 5056–5058.
- 10T. Kitamura, P. J. Stang, Tetrahedron Lett. 1988, 29, 1887–1890.
- 11I. F. D. Hyatt, M. P. Croatt, Angew. Chem. 2012, 124, 7629–7632;
10.1002/ange.201203062 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 7511–7514.
- 12M. Ochiai, M. Kunishima, K. Fuji, Y. Nagao, J. Org. Chem. 1988, 53, 6144–6145.
- 13
- 13aS. G. D’yachkova, E. A. Nikitina, N. K. Gusarova, A. I. Albanov, B. A. Trofimov, Russ. J. Gen. Chem. 2003, 73, 782–785;
- 13bR. Tanaka, K. Yamabe, J. Chem. Soc. Chem. Commun. 1983, 329–330.
- 14
- 14aK. Banert, R. Arnold, M. Hagedorn, P. Thoss, A. A. Auer, Angew. Chem. 2012, 124, 7633–7636; Angew. Chem. Int. Ed. 2012, 51, 7515–7518;
- 14bE. Prochnow, A. A. Auer, K. Banert, J. Phys. Chem. A 2007, 111, 9945–9951.