Electron Acceptors Based on an All-Carbon Donor–Acceptor Copolymer†
Jessica L. Jellison
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorChe-Hsiung Lee
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorXinju Zhu
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorJordan D. Wood
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorCorresponding Author
Prof. Kyle N. Plunkett
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.comSearch for more papers by this authorJessica L. Jellison
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorChe-Hsiung Lee
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorXinju Zhu
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorJordan D. Wood
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Search for more papers by this authorCorresponding Author
Prof. Kyle N. Plunkett
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.com
Department of Chemistry and Biochemistry, Southern Illinois University, Carbondale, IL (USA) http://www.kyleplunkett.comSearch for more papers by this authorThis work was supported by startup funds and a seed grant provided by Southern Illinois University.
Graphical Abstract
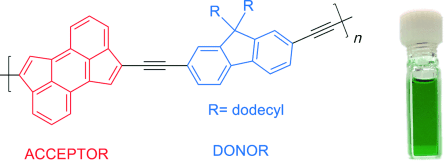
The Sonogashira cross-coupling polymerization of a dibrominated cyclopenta[hi]aceanthrylene and a diethynylfluorene derivative produced a donor–acceptor copolymer composed solely of cyclopenta-fused polycyclic aromatic hydrocarbons. The resulting polymer displays low band gaps (<1.5 eV), dual absorption bands, and electron-accepting behavior as demonstrated through fluorescence quenching of poly(3-hexylthiophene).
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206145_sm_miscellaneous_information.pdf1.1 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1E. E. Havinga, W. ten Hoeve, H. Wynberg, Synth. Met. 1993, 55, 299–306.
- 2aB. C. Thompson, J. M. J. Fréchet, Angew. Chem. 2008, 120, 62–82;
10.1002/ange.200702506 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 58–77;
- 2bM. Zhang, H. N. Tsao, W. Pisula, C. Yang, A. K. Mishra, K. Müllen, J. Am. Chem. Soc. 2007, 129, 3472–3473;
- 2cC. Li, M. Liu, N. G. Pschirer, M. Baumgarten, K. Müllen, Chem. Rev. 2010, 110, 6817–6855;
- 2dA. Facchetti, Chem. Mater. 2011, 23, 733–758.
- 3aC. J. Brabec, C. Winder, N. S. Sariciftci, J. C. Hummelen, A. Dhanabalan, P. A. van Hal, R. A. J. Janssen, Adv. Funct. Mater. 2002, 12, 709–712;
- 3bJ. Peet, J. Y. Kim, N. E. Coates, W. L. Ma, D. Moses, A. J. Heeger, G. C. Bazan, Nat. Mater. 2007, 6, 497–500.
- 4aA. Facchetti, M. Mushrush, M.-H. Yoon, G. R. Hutchison, M. A. Ratner, T. J. Marks, J. Am. Chem. Soc. 2004, 126, 13859–13874;
- 4bY. Wang, M. D. Watson, Macromolecules 2008, 41, 8643–8647.
- 5aX. Guo, F. S. Kim, S. A. Jenekhe, M. D. Watson, J. Am. Chem. Soc. 2009, 131, 7206–7207;
- 5bF. S. Kim, X. Guo, M. D. Watson, S. A. Jenekhe, Adv. Mater. 2010, 22, 478–482;
- 5cH. Yan, Z. Chen, Y. Zheng, C. Newman, J. R. Quinn, F. Dötz, M. Kastler, A. Facchetti, Nature 2009, 457, 679–686.
- 6L. Gao, W. Senevirathna, G. Sauvé, Org. Lett. 2011, 13, 5354–5357.
- 7J. D. Wood, J. L. Jellison, A. D. Finke, L. Wang, K. N. Plunkett, J. Am. Chem. Soc. 2012, 134, 15783–15789.
- 8aB. D. Steinberg, E. A. Jackson, A. S. Filatov, A. Wakamiya, M. A. Petrukhina, L. T. Scott, J. Am. Chem. Soc. 2009, 131, 10537–10545;
- 8bA. Ayalon, A. Sygula, P.-C. Cheng, M. Rabinovitz, P. W. Rabideau, L. T. Scott, Science 1994, 265, 1065–1067.
- 9aH. Reisch, U. Wiesler, U. Scherf, N. Tuytuylkov, Macromolecules 1996, 29, 8204–8210;
- 9bE. J. Meijer, D. M. De Leeuw, S. Setayesh, E. V. Veenendaal, B.-H. Huisman, P. W. M. Blom, J. C. Hummelen, U. Scherf, T. M. Klapwijk, Nat. Mater. 2003, 2, 678–682.
- 10aA. R. Mohebbi, J. Yuen, J. Fan, C. Munoz, M. F. Wang, R. S. Shirazi, J. Seifter, F. Wudl, Adv. Mater. 2011, 23, 4644–4648;
- 10bA. R. Mohebbi, F. Wudl, Chem. Eur. J. 2011, 17, 2642–2646.
- 11L. T. Scott, M. S. Bratcher, S. Hagen, J. Am. Chem. Soc. 1996, 118, 8743–8744.
- 12H. A. Wegner, L. T. Scott, A. de Meijere, J. Org. Chem. 2003, 68, 883–887.
- 13aD. T. Chase, B. D. Rose, S. P. McClintock, L. N. Zakharov, M. M. Haley, Angew. Chem. 2011, 123, 1159–1162; Angew. Chem. Int. Ed. 2011, 50, 1127–1130;
- 13bD. T. Chase, A. G. Fix, B. D. Rose, C. D. Weber, S. Nobusue, C. E. Stockwell, L. N. Zakharov, M. C. Lonergan, M. M. Haley, Angew. Chem. 2011, 123, 11299–11302;
10.1002/ange.201104797 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 11103–11106;
- 13cF. G. Brunetti, A. Varotto, N. A. Batara, F. Wudl, Chem. Eur. J. 2011, 17, 8604–8608.
- 14aT. Kawase, A. Konishi, Y. Hirao, K. Matsumoto, H. Kurata, T. Kubo, Chem. Eur. J. 2009, 15, 2653–2661;
- 14bM. Saito, M. Nakamura, T. Tajima, Chem. Eur. J. 2008, 14, 6062–6068.
- 15aH. Dang, M. A. Garcia-Garibay, J. Am. Chem. Soc. 2001, 123, 355–356;
- 15bH. Dang, M. Levitus, M. A. Garcia-Garibay, J. Am. Chem. Soc. 2002, 124, 136–143;
- 15cC. L. Eversloh, Y. Avlasevich, C. Li, K. Müllen, Chem. Eur. J. 2011, 17, 12756–12762;
- 15dH. Xia, D. Liu, X. Xu, Q. Miao, Chem. Commun. 2012, DOI: .
- 16T. Okamoto, Z. Bao, J. Am. Chem. Soc. 2007, 129, 10308–10309.
- 17P. M. Beaujuge, C. M. Amb, J. R. Reynolds, Acc. Chem. Res. 2010, 43, 1396–1407.
- 18aG. Sonmez, C. K. F. Shen, Y. Rubin, F. Wudl, Angew. Chem. 2004, 116, 1524–1528;
10.1002/ange.200352910 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 1498–1502;
- 18bG. Sonmez, H. B. Sonmez, C. K. F. Shen, R. W. Jost, Y. Rubin, F. Wudl, Macromolecules 2005, 38, 669–675.
- 19B. F. Plummer, M. J. Hopkinson, J. H. Zoeller, J. Am. Chem. Soc. 1979, 101, 6779–6781.
- 20aS. A. Jenekhe, L. Lu, M. M. Alam, Macromolecules 2001, 34, 7315–7324;
- 20bG. L. Gibson, T. M. McCormick, D. S. Seferos, J. Am. Chem. Soc. 2012, 134, 539–547.