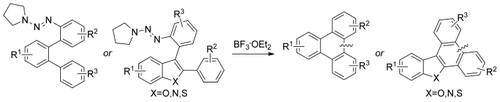Friedel–Crafts Arylation for the Formation of C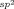 C
C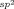 Bonds: A Route to Unsymmetrical and Functionalized Polycyclic Aromatic Hydrocarbons from Aryl Triazenes†
Bonds: A Route to Unsymmetrical and Functionalized Polycyclic Aromatic Hydrocarbons from Aryl Triazenes†
Jun Zhou
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Search for more papers by this authorWeijun Yang
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Search for more papers by this authorBinjie Wang
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Hongjun Ren
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Department of Chemistry, Zhejiang Sci-Tech University, Hangzhou 310018 (P. R. China)
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)Search for more papers by this authorJun Zhou
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Search for more papers by this authorWeijun Yang
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Search for more papers by this authorBinjie Wang
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Search for more papers by this authorCorresponding Author
Prof. Dr. Hongjun Ren
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)
Department of Chemistry, Zhejiang Sci-Tech University, Hangzhou 310018 (P. R. China)
Department of Chemistry, Zhejiang University, 38 Zheda Road, Hangzhou 310027 (P. R. China)Search for more papers by this authorWe are grateful for financial support from the National Nature Science Foundation of China (21272003) as well as a Scholarship Award for Excellent Doctoral Student granted by the Ministry of Education (2011).
Graphical Abstract
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206578_sm_miscellaneous_information.pdf1.4 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aA. J. Berresheim, M. Müller, K. Müllen, Chem. Rev. 1999, 99, 1747–1785;
- 1bM. Randić, Chem. Rev. 2003, 103, 3449–3605;
- 1cR. A. Pascal, Jr., Chem. Rev. 2006, 106, 4809–4819;
- 1dJ. Wu, W. Pisula, K. Müllen, Chem. Rev. 2007, 107, 718–747;
- 1eF. Chen, N. J. Tao, Acc. Chem. Res. 2009, 42, 429–438;
- 1fT. M. Figueira-Duarte, K. Müllen, Chem. Rev. 2011, 111, 7260–7314.
- 2
- 2a Metal-Catalyzed Cross-Coupling Reactions, Vol. 1, 2nd Completely Revised and Enlarged ed. ), Wiley-VCH, Weinheim, 2004;
- 2b Metal-Catalyzed Cross-Coupling Reactions, Vol. 2, 2nd Completely Revised and Enlarged ed. ), Wiley-VCH, Weinheim, 2004;
- 2c Metal-Catalyzed Cross-Coupling Reactions (Eds.: ), Wiley-VCH, Weinheim, 1998;
- 2dD. Alberico, M. E. Scott, M. Lautens, Chem. Rev. 2007, 107, 174–238.
- 3
- 3aL. Wang, P. B. Shevlin, Org. Lett. 2000, 2, 3703–3705;
- 3bX. H. Cheng, S. Höger, D. Fenske, Org. Lett. 2003, 5, 2587–2589;
- 3cH. A. Wegner, H. Reisch, K. Rauch, A. Demeter, K. A. Zachariasse, A. de Meijere, L. T. Scott, J. Org. Chem. 2006, 71, 9080–9087;
- 3dD. Kim, J. L. Petersen, K. K. Wang, Org. Lett. 2006, 8, 2313–2316;
- 3eE. A. Jackson, B. D. Steinberg, M. Bancu, A. Wakamiya, L. T. Scott, J. Am. Chem. Soc. 2007, 129, 484–485;
- 3fC.-W. Li, C.-I. Wang, H.-Y. Liao, R. Chaudhuri, R.-S. Liu, J. Org. Chem. 2007, 72, 9203–9207;
- 3gT.-A. Chen, R.-S. Liu, Org. Lett. 2011, 13, 4644–4647;
- 3hT.-C. Wu, H.-J. Hsin, M.-Y. Kuo, C.-H. Li, Y.-T. Wu, J. Am. Chem. Soc. 2011, 133, 16319–16321.
- 4
- 4aD. Peña, S. Escudero, D. Pérez, E. Guitián, L. Castedo, Angew. Chem. 1998, 110, 2804–2806;
10.1002/(SICI)1521-3757(19981002)110:19<2804::AID-ANGE2804>3.0.CO;2-2 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2659–2660;10.1002/(SICI)1521-3773(19981016)37:19<2659::AID-ANIE2659>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 4bZ. Liu, X. Zhang, R. C. Larock, J. Am. Chem. Soc. 2005, 127, 15716–15717;
- 4cT. T. Jayanth, C.-H. Cheng, Chem. Commun. 2006, 894–896;
- 4dZ. Liu, R. C. Larock, J. Org. Chem. 2007, 72, 223–232;
- 4eC. Xie, Y. Zhang, Z. Huang, P. Xu, J. Org. Chem. 2007, 72, 5431–5434;
- 4fC. Romero, D. Peña, D. Pérez, E. Guitián, Chem. Eur. J. 2006, 12, 5677–5684.
- 5
- 5aC. L. Hilton, C. R. Jamison, B. T. King, J. Am. Chem. Soc. 2006, 128, 14824;
- 5bX. Xue, L. T. Scott, Org. Lett. 2007, 9, 3937–3940;
- 5cM. Shimizu, I. Nagao, Y. Tomioka, T. Hiyama, Angew. Chem. 2008, 120, 8216–8219; Angew. Chem. Int. Ed. 2008, 47, 8096–8099;
- 5dI. Nagao, M. Shimizu, T. Hiyama, Angew. Chem. 2009, 121, 7709–7712;
10.1002/ange.200903779 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 7573–7576.
- 6
- 6aK. Mochida, K. Kawasumi, Y. Segawa, K. Itami, J. Am. Chem. Soc. 2011, 133, 10716–10719;
- 6bK. Kawasumi, K. Mochida, T. Kajino, Y. Segawa, K. Itami, Org. Lett. 2012, 14, 418–421.
- 7
- 7aA. McIver, D. D. Young, A. Deiters, Chem. Commun. 2008, 4750–4752;
- 7bC. Wang, S. Rakshit, F. Glorius, J. Am. Chem. Soc. 2010, 132, 14006–14008;
- 7cP. M. Donovan, L. T. Scott, J. Am. Chem. Soc. 2004, 126, 3108–3112;
- 7dA. Matsumoto, L. Ilies, E. Nakamura, J. Am. Chem. Soc. 2011, 133, 6557–6559;
- 7eM. Saifuddin, P. K. Agarwal, B. Kundu, J. Org. Chem. 2011, 76, 10122–10128.
- 8
- 8aH. Naarmann, M. Hanack, R. Mattmer, Synthesis 1994, 477–478;
- 8bX. Yang, X. Dou, K. Müllen, Chem. Asian J. 2008, 3, 759–766;
- 8cR. C. Borner, R. F. W. Jackson, J. Chem. Soc. Chem. Commun. 1994, 845–846;
- 8dS. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hägele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel, M. Tosoni, Angew. Chem. 2007, 119, 4916–4973;
10.1002/ange.200604203 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 4832–4887;
- 8eA. Fechtenkötter, K. Saalwächter, M. A. Harbison, K. Müllen, H. W. Spiess, Angew. Chem. 1999, 111, 3224–3228;
10.1002/(SICI)1521-3757(19991018)111:20<3224::AID-ANGE3224>3.0.CO;2-D Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3039–3042;10.1002/(SICI)1521-3773(19991018)38:20<3039::AID-ANIE3039>3.0.CO;2-5 CAS PubMed Web of Science® Google Scholar
- 8fX. Feng, J. Wu, V. Enkemann, K. Müllen, Org. Lett. 2006, 8, 1145–1148;
- 8gL. Zhai, R. Shukla, S. H. Wadumethrige, R. Rathore, J. Org. Chem. 2010, 75, 4748–4760;
- 8hL. Zhai, R. Shukla, R. Rathore, Org. Lett. 2009, 11, 3474–3477;
- 8iB. N. Boden, A. Abdolmaleki, C. T.-Z. Ma, M. J. MacLachlan, Can. J. Chem. 2008, 86, 50–64;
- 8jH. Meier, B. Rose, J. Prakt. Chem. 1998, 340, 536–543;
- 8kR. J. Bushby, C. Hardy, J. Chem. Soc. Perkin Trans. 1 1986, 721–723;
- 8lT. Sato, Y. Goto, K. Hata, Bull. Chem. Soc. Jpn. 1967, 40, 1994–1995;
- 8mT. Sato, S. Shigeru, K. Hata, Bull. Chem. Soc. Jpn. 1969, 42, 766–772;
- 8nT. Sato, S. Shimada, K. Hata, Bull. Chem. Soc. Jpn. 1971, 44, 2484–2490;
- 8oN. Kharasch, T. G. Alston, H. B. Lewis, W. Wolf, Chem. Commun. 1965, 242–243.
- 9
- 9aW. Y. Lee, W. Sim, H.-J. Kim, S.-H. Yoon, J. Chem. Soc. Perkin Trans. 1 1993, 719–729;
- 9bJ. Ichikawa, M. Yokota, T. Kudo, S. Umezaki, Angew. Chem. 2008, 120, 4948–4951;
10.1002/ange.200801396 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4870–4873;
- 9cD. Ferraris, C. Cox, R. Anand, T. Lectka, J. Am. Chem. Soc. 1997, 119, 4319–4320.
- 10
- 10aM. Fagnoni, A. Albini, Acc. Chem. Res. 2005, 38, 713–721;
- 10bY. Apeloig, D. Arad, J. Am. Chem. Soc. 1985, 107, 5285–5286;
- 10cV. Dichiarante, A. Salvaneschi, S. Protti, D. Dondi, M. Fagnoni, A. Albini, J. Am. Chem. Soc. 2007, 129, 15919–15926;
- 10dS. Lazzaroni, D. Dondi, M. Fagnoni, A. Albini, J. Org. Chem. 2010, 75, 315–323;
- 10eA. Nicolaides, D. M. Smith, F. Jensen, L. Radom, J. Am. Chem. Soc. 1997, 119, 8083–8088.
- 11O. Allemann, S. Duttwyler, P. Romanato, K. K. Baldridge, J. S. Siegel, Science 2011, 332, 574–577.
- 12For examples of aryl–aryl coupling by HF elimination, see:
- 12aK. Y. Amsharov, M. A. Kabdulov, M. Jansen, Angew. Chem. 2012, 124, 4672–4675;
10.1002/ange.201200516 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 4594–4597;
- 12bK. Y. Amsharov, M. A. Kabdulov, M. Jansen, Chem. Eur. J. 2010, 16, 5868–5871;
- 12cK. Y. Amsharov, P. Merz, J. Org. Chem. 2012, 77, 5445–5448.
- 13
- 13aH. Zollinger, Acc. Chem. Res. 1973, 6, 335–341;
- 13bR. G. Bergstrom, R. G. M. Landells, G. H. Wahl, Jr., H. Zollinger, J. Am. Chem. Soc. 1976, 98, 3301–3305;
- 13cI. Szele, H. Zollinger, J. Am. Chem. Soc. 1978, 100, 2811–2815;
- 13dY. Hashida, R. G. M. Landells, G. E. Lewis, I. Szele, H. Zollinger, J. Am. Chem. Soc. 1978, 100, 2816–2823;
- 13eR. Glaser, C. J. Horan, M. Lewis, H. Zollinger, J. Org. Chem. 1999, 64, 902–913.
- 14
- 14aM. Gomberg, W. E. Bachmann, J. Am. Chem. Soc. 1924, 46, 2339–2343;
- 14bV. Meille, E. Schulz, M. Lemaire, R. Faure, M. Vrinat, Tetrahedron 1996, 52, 3953–3960;
- 14cS. A. Chandler, P. Hanson, A. B. Taylor, P. H. Walton, A. W. Timms, J. Chem. Soc. Perkin Trans. 2 2001, 214–228;
- 14dJ. Forrest, J. Chem. Soc. 1960, 581–588;
- 14eJ. Forrest, J. Chem. Soc. 1960, 594–601;
- 14fT. D. Tuong, M. Hida, J. Chem. Soc. Perkin Trans. 2 1974, 676–682;
- 14gJ. Hassan, M. Sévignon, C. Gozzi, E. Schulz, M. Lemaire, Chem. Rev. 2002, 102, 1359–1469.
- 15
- 15aT. Saeki, E.-C. Son, K. Tamao, Org. Lett. 2004, 6, 617–619;
- 15bT. B. Patrick, R. P. Willaredt, D. J. Degonia, J. Org. Chem. 1985, 50, 2232–2235;
- 15cT. Saeki, T. Matsunaga, E.-C. Son, K. Tamao, Adv. Synth. Catal. 2004, 346, 1689–1692;
- 15dC.-Y. Liu, A. Gavryushin, P. Knochel, Chem. Asian J. 2007, 2, 1020–1030.
- 16
- 16aW. Yang, J. Zhou, B. Wang, H. Ren, Chem. Eur. J. 2011, 17, 13665–13669;
- 16bG. Zhao, B. Wang, W. Yang, H. Ren, Eur. J. Org. Chem. 2012, 31, 6236–6247.
- 17
- 17aJ. M. Quimby, L. T. Scott, Adv. Synth. Catal. 2009, 351, 1009–1013;
- 17bH. Yoshida, K. Okada, S. Kawashima, K. Tanino, J. Ohshita, Chem. Commun. 2010, 46, 1763–1765;
- 17cH. A. Wegner, L. T. Scott, A. de Meijere, J. Org. Chem. 2003, 68, 883–887;
- 17dQ. Huang, M. A. Campo, T. Yao, Q. Tian, R. C. Larock, J. Org. Chem. 2004, 69, 8251–8257;
- 17eJ. E. Rice, Z.-W. Cai, Tetrahedron Lett. 1992, 33, 1675–1678;
- 17fJ. E. Rice, Z.-W. Cai, Z.-M. He, E. J. LaVoie, J. Org. Chem. 1995, 60, 8101–8104;
- 17gJ. E. Rice, Z.-W. Cai, J. Org. Chem. 1993, 58, 1415–1424;
- 17hX. Chen, P. Lu, Y. Wang, Chem. Eur. J. 2011, 17, 8105–8114.
- 18D. G. Pintori, M. F. Greaney, J. Am. Chem. Soc. 2011, 133, 1209–1211.
- 19J. H. Delcamp, A. Yella, T. W. Holcombe, M. K. Nazeeruddin, M. Grätzel, Angew. Chem. 2012, DOI: ; Angew. Chem. Int. Ed. 2012, DOI: .
- 20
- 20aS. H. Cho, J. Yoon, S. Chang, J. Am. Chem. Soc. 2011, 133, 5996–6005;
- 20bJ. Zhao, Y. Zhao, H. Fu, Angew. Chem. 2011, 123, 3853–3857; Angew. Chem. Int. Ed. 2011, 50, 3769–3773.
- 21Recently, Sanford and co-workers developed a mild arylation reaction using aryldiazonium salts in the presence of Pd and Ru; see: D. Kalyani, K. B. McMurtrey, S. R. Neufeldt, M. S. Sanford, J. Am. Chem. Soc. 2011, 133, 18566–18569.