CF Bond Activation of Unactivated Aliphatic Fluorides: Synthesis of Fluoromethyl-3,5-diaryl-2-oxazolidinones by Desymmetrization of 2-Aryl-1,3-difluoropropan-2-ols†
Corresponding Author
Prof. Dr. Günter Haufe
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, 48149 Münster (Germany)
Günter Haufe, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, 48149 Münster (Germany)
Norio Shibata, Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorSatoru Suzuki
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorHiroyuki Yasui
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorChisato Terada
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorTakashi Kitayama
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorDr. Motoo Shiro
Rigaku Corporation, 3-9-12 Matsubara-cho, Akishima, Tokyo 196-8666 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Norio Shibata
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Günter Haufe, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, 48149 Münster (Germany)
Norio Shibata, Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Günter Haufe
Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, 48149 Münster (Germany)
Günter Haufe, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, 48149 Münster (Germany)
Norio Shibata, Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorSatoru Suzuki
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorHiroyuki Yasui
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorChisato Terada
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorTakashi Kitayama
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorDr. Motoo Shiro
Rigaku Corporation, 3-9-12 Matsubara-cho, Akishima, Tokyo 196-8666 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Norio Shibata
Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Günter Haufe, Organisch-Chemisches Institut, Westfälische Wilhelms-Universität Münster, 48149 Münster (Germany)
Norio Shibata, Department of Frontier Materials, Nagoya Institute of Technology, Gokiso, Showa-ku, Nagoya 466-8555 (Japan)
Search for more papers by this authorThis study was financially supported in part by Grants-in-Aid for Scientific Research from MEXT (Ministry of Education, Culture, Sports, Science and Technology) (24105513, Project No. 2304: Advanced Molecular Transformation by Organocatalysts) and by the Deutsche Forschungsgemeinschaft (DFG). We thank the Asahi Glass Foundation for partial support.
Graphical Abstract
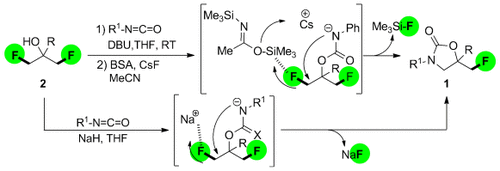
How to lose fluorine: Biologically relevant oxazolidinones 1 were synthesized through the desymmetrization of unactivated aliphatic difluorides by Si-induced catalytic CF bond-cleavage using BSA/CsF (BSA=bis(trimethylsilyl)acetamide). The direct transformation of 2 with isocyanates into 1 by cascade carbamoylation/cyclization, in which cyclization is induced by Na-assisted CF bond activation, was also achieved.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201207304_sm_miscellaneous_information.pdf13.7 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aT. Hiyama, Organofluorine Compounds. Chemistry and Applications, Springer, Berlin, 2000;
10.1007/978-3-662-04164-2 Google Scholar
- 1bP. Kirsch, Modern Fluoroorganic Chemistry, Wiley-VCH, Weinheim, 2004;
10.1002/352760393X Google Scholar
- 1cR. D. Chambers, Fluorine in Organic Chemistry, Blackwell, Oxford, 2004;
10.1002/9781444305371 Google Scholar
- 1dK. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006;
10.1002/9780470988589 Google Scholar
- 1e Handbook of Fluorous Chemistry (Eds.: ), Wiley-VCH, Weinheim, 2004;
- 1fZ. Hong, S. G. Weber Teflon AF Materials in Topics in Current Chemistry, Vol. 308 (Ed.: ), Springer, Berlin, 2012, pp. 307–377.
- 2
- 2aR. E. Banks, D. W. A. Sharp, J. C. Tatlow, Fluorine—The First Hundred Years (1886–1986), Elsevier, New York, 1986;
- 2b Organofluorine Chemistry. Principles and Commercial Applications (Eds.: ), Plenum, New York, 1994;
- 2cM. Hudlický, A. E. Pavlath, Chemistry of Organic Fluorine Compounds II, A Critical Review, ACS Monograph 187, American Chemical Society, Washington, 1995.
- 3
- 3aH. Amii, K. Uneyama, Chem. Rev. 2009, 109, 2119–2183;
- 3bG. K. Surya Prakash, J. Hu, G. A. Olah, J. Fluorine Chem. 2001, 112, 355–360;
10.1016/S0022-1139(01)00535-8 Google Scholar
- 3cS. Higashiya, W. J. Chung, D. S. Lim, S. C. Ngo, W. H. Kelly IV, P. J. Toscano, J. T. Welch, J. Org. Chem. 2004, 69, 6323–6328;
- 3dK. Fuchibe, Y. Ohshima, K. Mitomi, T. Akiyama, Org. Lett. 2007, 9, 1497–1499;
- 3eH. Yanai, T. Taguchi, Chem. Commun. 2009, 1034–1036;
- 3fJ. Wettergren, T. Ankner, G. Hilmersson, Chem. Commun. 2010, 46, 7596–7597;
- 3gH. Yanai, T. Taguchi, Tetrahedron 2010, 66, 4530–4541;
- 3hM. F. Kühnel, D. Lentz, Angew. Chem. 2010, 122, 2995–2998;
10.1002/ange.200907162 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 2933–2936.
- 4
- 4aS. J. Blanksby, G. B. Ellison, Acc. Chem. Res. 2003, 36, 255–263;
- 4bD. O’Hagan, Chem. Soc. Rev. 2008, 37, 308–319.
- 5
- 5aY. Kiso, K. Tamao, M. Kumada, J. Organomet. Chem. 1973, 50, C 12–C14;
- 5bT. G. Richmond, Angew. Chem. 2000, 112, 3378–3380;
10.1002/1521-3757(20000915)112:18<3378::AID-ANGE3378>3.0.CO;2-A Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3241–3244;10.1002/1521-3773(20000915)39:18<3241::AID-ANIE3241>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 5cR. N. Perutz, T. Braun, Transition-Metal Mediated C-F Bond Activation in Comprehensive Organometallic Chemistry III, Vol. 1 (Eds.: ), Elsevier, Amsterdam, 2007, pp. 725–758;
- 5dH. Torrens, Coord. Chem. Rev. 2005, 249, 1957–1985;
- 5efor a special issue on CF bond activation, see: T. Braun, Organometallic Chemistry Meets Fluorine in Organometallics 2012, 31, 1213–1215.
- 6
- 6aE. D. Bergmann, A. Mielewitz, J. Chem. Soc. 1963, 3736–3739;
- 6bY. Avi-Dor, J. Mager, J. Biol. Chem. 1956, 222, 249–258;
- 6cM. E. Tanner, S. Miao, Tetrahedron Lett. 1994, 35, 4073–4076;
- 6dF. Rolla, J. Org. Chem. 1981, 46, 3909–3911;
- 6eT. Imamoto, T. Takeyama, T. Kusumoto, Chem. Lett. 1985, 14, 1491–1492;
- 6fD. Guijarro, M. Yus, J. Organomet. Chem. 2001, 624, 53–57;
- 6gM. G. Saulnier, D. R. Langley, D. B. Frennesson, B. H. Long, S. Huang, Q. Gao, D. Wu, C. R. Fairchild, E. Ruediger, K. Zimmermann, D. R. St. Laurent, B. N. Balasubramanian, D. M. Vyas, Org. Lett. 2005, 7, 1271–1274;
- 6hJ. Ichikawa, G. Lapointe, Y. Iwai, Chem. Commun. 2007, 2698–2700.
- 7
- 7aK. Mikami, Y. Tomita, Y. Itoh, Angew. Chem. 2010, 122, 3907–3910;
10.1002/ange.201000435 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 3819–3822;
- 7bK. Matsubara, T. Ishibashi, Y. Koga, Org. Lett. 2009, 11, 1765–1768;
- 7cW. Gu, M. R. Haneline, C. Douvris, O. V. Ozerov, J. Am. Chem. Soc. 2009, 131, 11203–11212;
- 7dC. B. Caputo, D. W. Stephen, Organometallics 2012, 31, 27–30;
- 7eD. L. Parker, Jr., A. K. Fried, D. Meng, M. L. Greenlee, Org. Lett. 2008, 10, 2983–2985;
- 7fA. Hazari, V. Gouverneur, J. M. Brown, Angew. Chem. 2009, 121, 1322–1325;
10.1002/ange.200804310 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1296–1299;
- 7gG. Meier, M. Braun, Angew. Chem. 2009, 121, 1575–1577;
10.1002/ange.200805237 Google ScholarAngew. Chem. Int. Ed. 2009, 48, 1546–1548;
- 7hE. Benedetto, M. Keita, M. Tredwell, C. Hollingworth, J. M. Brown, V. Gouverneur, Organometallics 2012, 31, 1408–1416;
- 7iG. Blessley, P. Holden, M. Walker, J. M. Brown, V. Gouverneur, Org. Lett. 2012, 14, 2754–2757;
- 7jA. Nova, R. Mas-Ballesté, G. Ujaque, P. González-Duarte, A. Lledós, Chem. Commun. 2008, 3130–3132;
- 7kX. Pigeon, M. Bergeron, F. Barabé, P. Dubé, H. N. Frost, J.-F. Paquin, Angew. Chem. 2010, 122, 1141–1145;
10.1002/ange.200904747 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 1123–1127;
- 7lT. Iida, R. Hashimoto, K. Aikawa, S. Ito, K. Mikami, Angew. Chem. 2012, 124, 9673–9676;
10.1002/ange.201203588 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 9535–9538; see also Ref. [3a] for references prior to 2009.
- 8
- 8aJ. Choi, D. Y. Wang, S. Kundu, Y. Choliy, T. J. Emge, K. Krogh-Jespersen, A. S. Goldman, Science 2011, 332, 1545–1548;
- 8bN. Lühmann, R. Panisch, T. Müller, Appl. Organomet. Chem. 2010, 24, 533–537;
- 8cD. Guijarro, P. Martínez, M. Yus, Tetrahedron 2003, 59, 1237–1244;
- 8dC. Melero, R. P. Herrera, A. Guijarro, M. Yus, Chem. Eur. J. 2007, 13, 10096–10107;
- 8eL. Zhang, W. Zhang, J. Liu, J. Hu, J. Org. Chem. 2009, 74, 2850–2853;
- 8fT. Hatakeyama, S. Ito, H. Yamane, M. Nakamura, E. Nakamura, Tetrahedron 2007, 63, 8440–8448;
- 8gT. Ooi, D. Uraguchi, N. Kagoshima, K. Maruoka, Tetrahedron Lett. 1997, 38, 5679–5682;
- 8hK. Tani, K. Suwa, T. Yamagata, S. Otsuka, Chem. Lett. 1982, 265–268.
- 9A. Y. Rulev, V. M. Muzalevskiy, E. V. Kondrashov, I. A. Ushakov, A. S. Shastin, E. S. Balenkova, G. Haufe, V. G. Nenajdenko, Eur. J. Org. Chem. 2010, 300–310.
- 10
- 10aT. C. Rosen, S. Yoshida, R. Fröhlich, K. L. Kirk, G. Haufe, J. Med. Chem. 2004, 47, 5860–5871;
- 10bT. C. Rosen, S. Yoshida, K. L. Kirk, G. Haufe, ChemBioChem 2004, 5, 1033–1043;
- 10cJ. Oldendorf, G. Haufe, Eur. J. Org. Chem. 2006, 4463–4472;
- 10dA. K. Podichetty, S. Wagner, S. Schröer, A. Faust, M. Schäfers, O. Schober, K. Kopka, G. Haufe, J. Med. Chem. 2009, 52, 3484–3495;
- 10eA. K. Podichetty, S. Wagner, A. Faust, M. Schäfers, O. Schober, K. Kopka, G. Haufe, Future Med. Chem. 2009, 1, 969–989;
- 10fK. Koroniak, G. Haufe, Synthesis 2010, 3309–3314;
- 10gD. Schrigten, H.-J. Breyholz, S. Wagner, S. Hermann, O. Schober, M. Schäfers, G. Haufe, K. Kopka, J. Med. Chem. 2012, 55, 223–232.
- 11
- 11aK. Matoba, H. Kawai, T. Furukawa, A. Kusuda, E. Tokunaga, S. Nakamura, M. Shiro, N. Shibata, Angew. Chem. 2010, 122, 5898–5902;
10.1002/ange.201002065 Google ScholarAngew. Chem. Int. Ed. 2010, 49, 5762–5766;
- 11bH. Kawai, K. Tachi, E. Tokunaga, M. Shiro, N. Shibata, Angew. Chem. 2011, 123, 7949–7952; Angew. Chem. Int. Ed. 2011, 50, 7803–7806;
- 11cH. Kawai, S. Okusu, E. Tokunaga, H. Sato, M. Shiro, N. Shibata, Angew. Chem. 2012, 124, 5043–5046; Angew. Chem. Int. Ed. 2012, 51, 4959–4962.
- 12
- 12aR. B. Fugitt, R. W. Luckenbaugh, US4128654, 1978;
- 12bD. M. Chan, J. K. Long, WO2007123853, 2007;
- 12cH. Ihara, K. Kumamoto, WO2010090344, 2010.
- 13The result of a substructure search for 2 using the SciFinder database in August, 2012.
- 14 CRC Handbook of Chemistry and Physics, 91st ed. ), CRC, Boca Raton, FL, 2010–2011.
- 15A similar transformation has been reported by Hu et.al.,[8e] although they do not mention any assistance from NaF.
- 16CCDC-898314 (1 d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.