Triple-Decker Au3–Ag–Au3–Ag–Au3 Ion Cluster Enclosed in a Self-Assembled Cage†
Takafumi Osuga
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Takashi Murase
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Fujita
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorTakafumi Osuga
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorDr. Takashi Murase
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Search for more papers by this authorCorresponding Author
Prof. Dr. Makoto Fujita
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)
Department of Applied Chemistry, School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656 (Japan)Search for more papers by this authorThis work was supported by a CREST (Core Research for Evolution Science and Technology) project from the Japan Science and Technology Agency (JST).
Graphical Abstract
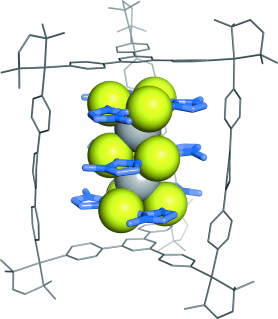
Silver in a gold mine: A triple-decker AuI–AgI ion cluster was synthesized by alternate alignment of cyclic trinuclear AuI complexes and AgI ions within a self-assembled cage. The box-shaped cavity of the cage is suitable not only for limiting the cluster number, but also for the stabilization of weakly associated metal ions, which cannot exist without the help of the cage.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201207619_sm_miscellaneous_information.pdf6.8 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For selected reviews, see:
- 1aP. Pyykkö, Chem. Rev. 1997, 97, 597–636;
- 1bC.-M. Che, S.-W. Lai, Coord. Chem. Rev. 2005, 249, 1296–1309;
- 1cH. Schmidbaur, A. Schier, Chem. Soc. Rev. 2008, 37, 1931–1951;
- 1dH. Schmidbaur, A. Schier, Chem. Soc. Rev. 2012, 41, 370–412.
- 2
- 2aM. J. Katz, K. Sakai, D. B. Leznoff, Chem. Soc. Rev. 2008, 37, 1884–1895;
- 2bO. Crespo in Modern Supermolecular Gold Chemistry: Gold-Metal Interactions and Applications (Ed.: ), Wiley-VCH, Weinheim, 2008, pp. 65–129.
10.1002/9783527623778.ch2 Google Scholar
- 3
- 3aB. K. Teo, K. Keating, J. Am. Chem. Soc. 1984, 106, 2224–2226;
- 3bO. Crespo, M. C. Gimeno, P. G. Jones, A. Laguna, M. D. Villacampa, Angew. Chem. 1997, 109, 1025–1027;
10.1002/ange.19971090921 Google ScholarAngew. Chem. Int. Ed. Engl. 1997, 36, 993–995.
- 4T. Osuga, T. Murase, K. Ono, Y. Yamauchi, M. Fujita, J. Am. Chem. Soc. 2010, 132, 15553–15555.
- 5
- 5aA. Burini, J. P. Fackler, Jr., R. Galassi, B. R. Pietroni, R. J. Staples, Chem. Commun. 1998, 95–96;
- 5bA. Burini, R. Bravi, J. P. Fackler, Jr., R. Galassi, T. A. Grant, M. A. Omary, B. R. Pietroni, R. J. Staples, Inorg. Chem. 2000, 39, 3158–3165.
- 6
- 6aM. Yoshizawa, J. Nakagawa, K. Kumazawa, M. Nagao, M. Kawano, T. Ozeki, M. Fujita, Angew. Chem. 2005, 117, 1844–1847; Angew. Chem. Int. Ed. 2005, 44, 1810–1813;
- 6bM. Yoshizawa, K. Ono, K. Kumazawa, T. Kato, M. Fujita, J. Am. Chem. Soc. 2005, 127, 10800–10801;
- 6cK. Ono, M. Yoshizawa, T. Kato, K. Watanabe, M. Fujita, Angew. Chem. 2007, 119, 1835–1838;
10.1002/ange.200604790 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 1803–1806;
- 6dK. Ono, M. Yoshizawa, T. Kato, M. Fujita, Chem. Commun. 2008, 2328–2330.
- 7
- 7aF. Bonati, A. Burini, B. R. Pietroni, J. Organomet. Chem. 1989, 375, 147–160;
- 7bG. Yang, R. G. Raptis, Inorg. Chem. 2003, 42, 261–263; for reviews of cyclic trinuclear AuI complexes, see:
- 7cA. A. Mohamed, J. P. Fackler, Jr., Comments Inorg. Chem. 2003, 24, 253–280;
- 7dH. E. Abdou, A. A. Mohamed, J. P. Fackler, Jr., in Gold Chemistry: Applications and Future Directions in the Life Science (Ed.: ), Wiley-VCH, Weinheim, 2008, pp. 1–45.
- 8
- 8aL. E. Sansores, R. Salcedo, A. Martínez, N. Mireles, THEOCHEM 2006, 763, 7–11;
- 8bS. M. Tekarli, T. R. Cundari, M. A. Omary, J. Am. Chem. Soc. 2008, 130, 1669–1675.
- 9In this study, we discussed the molecular geometry of the largest portion of disordered molecules. The Au⋅⋅⋅Ag distances are close to those observed in an infinite (Au3–Ag–Au3)n crystalline chain (2.731–2.922 Å), see ref. [5a].
- 10These Au⋅⋅⋅Ag distances are less than the sum of the van der Waals radii (3.4 Å); see:
- 10aA. Bondi, J. Phys. Chem. 1964, 68, 441–451; for Au⋅⋅⋅Ag interactions, see:
- 10bV. J. Catalano, A. L. Moor, Inorg. Chem. 2005, 44, 6558–6566;
- 10cA. A. Mohamed, A. Burini, J. P. Fackler, Jr., J. Am. Chem. Soc. 2005, 127, 5012–5013.
- 11The Au⋅⋅⋅Ag distances ranged from 2.774 to 2.818 Å.
- 12AuI–AgI ion clusters were not emissive, as cage 1 strongly absorbs light with λ<350 nm and host–guest interactions can also efficiently quench guest emission; see:
- 12aJ. K. Klosterman, M. Iwamura, T. Tahara, M. Fujita, J. Am. Chem. Soc. 2009, 131, 9478–9479;
- 12bK. Ono, J. K. Klosterman, M. Yoshizawa, K. Sekiguchi, T. Tahara, M. Fujita, J. Am. Chem. Soc. 2009, 131, 12526–12527.
- 13
- 13aJ. K. Klosterman, Y. Yamauchi, M. Fujita, Chem. Soc. Rev. 2009, 38, 1714–1725;
- 13bY. Yamauchi, M. Yoshizawa, M. Akita, M. Fujita, J. Am. Chem. Soc. 2010, 132, 960–966.