Rhodium(III)-Catalyzed Oxidative CH Coupling of N-Methoxybenzamides with Aryl Boronic Acids: One-Pot Synthesis of Phenanthridinones†
Jaganathan Karthikeyan
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorRadhakrishnan Haridharan
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorCorresponding Author
Prof. Dr. Chien-Hong Cheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7EchchengSearch for more papers by this authorJaganathan Karthikeyan
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorRadhakrishnan Haridharan
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Search for more papers by this authorCorresponding Author
Prof. Dr. Chien-Hong Cheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7Echcheng
Department of Chemistry, National Tsing Hua University, Hsinchu 30013 (Taiwan) http://mx.nthu.edu.tw/%7EchchengSearch for more papers by this authorWe thank the National Science Council of the Republic of China (NSC 98-2119-M-007-002-MY3) for support of this research.
Graphical Abstract
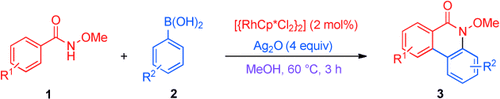
General solution: An efficient rhodium-catalyzed dual CH bond activation and cyclization of N-methoxybenzamides 1 with aryl boronic acids 2 (see scheme; Cp*=Me5C5) provides a straightforward and general approach to the phenanthridinone structure, which occurs widely in natural products and drugs. Highly regioselective CC and CN bond formation under mild conditions afforded a wide range of substituted phenanthridinones 3.
Supporting Information
Detailed facts of importance to specialist readers are published as ”Supporting Information”. Such documents are peer-reviewed, but not copy-edited or typeset. They are made available as submitted by the authors.
| Filename | Description |
|---|---|
| anie_201206890_sm_miscellaneous_information.pdf5.6 MB | miscellaneous_information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aL. Ackermann, R. Vicente, A. R. Kapdi, Angew. Chem. 2009, 121, 9976; Angew. Chem. Int. Ed. 2009, 48, 9792;
- 1bT. W. Lyons, M. S. Sanford, Chem. Rev. 2010, 110, 1147;
- 1cX. Chen, K. M. Engle, D.-H. Wang, J.-Q. Yu, Angew. Chem. 2009, 121, 5196; Angew. Chem. Int. Ed. 2009, 48, 5094;
- 1dO. Daugulis, H.-Q. Do, D. Shabashov, Acc. Chem. Res. 2009, 42, 1074;
- 1eJ. Wencel-Delord, T. Dröge, F. Liu, F. Glorius, Chem. Soc. Rev. 2011, 40, 4740; for reviews focused on oxidative coupling, see:
- 1fC. S. Yeung, V. M. Dong, Chem. Rev. 2011, 111, 1215;
- 1gC. Liu, H. Zhang, W. Shi, A. Lei, Chem. Rev. 2011, 111, 1780.
- 2
- 2aL. McMurray, F. O’Hara, M. J. Gaunt, Chem. Soc. Rev. 2011, 40, 1885;
- 2bC. Zhu, R. Wang, J. R. Falck, Chem. Asian J. 2012, 7, 1502.
- 3For reviews on rhodium-catalyzed CH activation, see:
- 3aD. A. Colby, R. G. Bergman, J. A. Ellman, Chem. Rev. 2010, 110, 624;
- 3bG. Song, F. Wang, X. Li, Chem. Soc. Rev. 2012, 41, 3651;
- 3cD. A. Colby, A. S. Tsai, R. G. Bergman, J. A. Ellman, Acc. Chem. Res. 2012, 45, 814.
- 4For selected examples of RhIII-catalyzed oxidative coupling with alkenes, see:
- 4aK. Ueura, T. Satoh, M. Miura, Org. Lett. 2007, 9, 1407;
- 4bN. Umeda, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2009, 74, 7094;
- 4cJ. Chen, G. Song, C.-L. Pan, X. Li, Org. Lett. 2010, 12, 5426;
- 4dF. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9982;
- 4eS. Mochida, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2011, 76, 3024;
- 4fX. Li, M. Zhao, J. Org. Chem. 2011, 76, 8530;
- 4gA. S. Tsai, M. Brasse, R. G. Bergman, J. A. Ellman, Org. Lett. 2011, 13, 540;
- 4hS. H. Park, J. Y. Kim, S. Chang, Org. Lett. 2011, 13, 2372;
- 4iF. W. Patureau, T. Besset, F. Glorius, Angew. Chem. 2011, 123, 1096; Angew. Chem. Int. Ed. 2011, 50, 1064;
- 4jH. Li, Y. Li, X.-S. Zhang, K. Chen, X. Wang, Z.-J. Shi, J. Am. Chem. Soc. 2011, 133, 15244;
- 4kS. Rakshit, C. Grohmann, T. Besset, F. Glorius, J. Am. Chem. Soc. 2011, 133, 2350.
- 5For selected examples of RhIII-catalyzed oxidative coupling with alkynes, see:
- 5aN. Umeda, H. Tsurugi, T. Satoh, M. Miura, Angew. Chem. 2008, 120, 4083;
10.1002/ange.200800924 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4019;
- 5bS. Mochida, N. Umeda, K. Hirano, T. Satoh, M. Miura, Chem. Lett. 2010, 39, 744;
- 5cP. C. Too, Y.-F. Wang, S. Chiba, Org. Lett. 2010, 12, 5688;
- 5dT. K. Hyster, T. Rovis, J. Am. Chem. Soc. 2010, 132, 10565;
- 5eS. Rakshit, F. W. Patureau, F. Glorius, J. Am. Chem. Soc. 2010, 132, 9585;
- 5fN. Umeda, K. Hirano, T. Satoh, N. Shibata, H. Sato, M. Miura, J. Org. Chem. 2011, 76, 13;
- 5gM. P. Huestis, L. Chan, D. R. Stuart, K. Fagnou, Angew. Chem. 2011, 123, 1374; Angew. Chem. Int. Ed. 2011, 50, 1338;
- 5hN. Guimond, S. I. Gorelsky, K. Fagnou, J. Am. Chem. Soc. 2011, 133, 6449;
- 5iF. W. Patureau, T. Besset, N. Kuhl, F. Glorius, J. Am. Chem. Soc. 2011, 133, 2154;
- 5jT. K. Hyster, T. Rovis, Chem. Commun. 2011, 47, 11846;
- 5kB.-J. Li, H.-Y. Wang, Q.-L. Zhu, Z.-J. Shi, Angew. Chem. 2012, 124, 4014; Angew. Chem. Int. Ed. 2012, 51, 3948;
- 5lK. Muralirajan, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2011, 123, 4255;
10.1002/ange.201100229 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 4169;
- 5mJ. Jayakumar, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2012, 124, 201;
10.1002/ange.201105755 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 197;
- 5nN. Guimond, C. Gouliaras, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 6908.
- 6
- 6aA. S. Tsai, M. E. Tauchert, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2011, 133, 1248;
- 6bY. Li, B.-J. Li, W.-H. Wang, W.-P. Huang, X.-S. Zhang, K. Chen, Z.-J. Shi, Angew. Chem. 2011, 123, 2163; Angew. Chem. Int. Ed. 2011, 50, 2115;
- 6cM. E. Tauchert, C. D. Incarvito, A. L. Rheingold, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2012, 134, 1482;
- 6dY. Li, X.-S. Zhang, H. Li, W.-H. Wang, K. Chen, B.-J. Li, Z.-J. Shi, Chem. Sci. 2012, 3, 1634;
- 6eY. Du, T. K. Hyster, T. Rovis, Chem. Commun. 2011, 47, 12074;
- 6fK. D. Hesp, R. G. Bergman, J. A. Ellman, J. Am. Chem. Soc. 2011, 133, 11430;
- 6gC. Zhu, R. Wang, J. R. Falck, Chem. Eur. J. 2011, 17, 12591;
- 6hJ. Park, E. Park, A. Kim, Y. Lee, K.-W. Chi, J. H. Kwak, Y. H. Jung, I. S. Kim, Org. Lett. 2011, 13, 4390;
- 6iL. Yang, C. A. Correia, C.-J. Li, Adv. Synth. Catal. 2011, 353, 1269;
- 6jY. Li, X.-S. Zhang, K. Chen, K.-H. He, F. Pan, B.-J. Li, Z.-J. Shi, Org. Lett. 2012, 14, 636.
- 7K. Chen, H. Li, Y. Li, X.-S. Zhang, Z.-Q. Lei, Z.-J. Shi, Chem. Sci. 2012, 3, 1645.
- 8J. Wencel-Delord, C. Nimphius, F. W. Patureau, F. Glorius, Angew. Chem. 2012, 124, 2290;
10.1002/ange.201107842 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 2247.
- 9For selected examples of Pd-catalyzed CH activation reactions with organoboron reagents, see:
- 9aX. Chen, J. J. Li, C. E. Goodhue, J. Q. Yu, J. Am. Chem. Soc. 2006, 128, 12634;
- 9bR. Giri, N. Maugel, J.-J. Li, D.-H. Wang, S. P. Breazzano, L. B. Saunders, J.-Q. Yu, J. Am. Chem. Soc. 2007, 129, 3510;
- 9cD. H. Wang, T. S. Mei, J.-Q. Yu, J. Am. Chem. Soc. 2008, 130, 17676;
- 9dS. D. Yang, C. L. Sun, Z. Fang, B. J. Li, Y.-Z. Li, Z.-J. Shi, Angew. Chem. 2008, 120, 1495; Angew. Chem. Int. Ed. 2008, 47, 1473;
- 9eC.-L. Sun, N. Liu, B.-J. Li, D.-G. Yu, Y. Wang, Z.-J. Shi, Org. Lett. 2010, 12, 184;
- 9fT. Nishikata, A. R. Abela, S. Huang, B. H. Lipshutz, J. Am. Chem. Soc. 2010, 132, 4978;
- 9gM. J. Tredwell, M. Gulias, N. G. Bremeyer, C. C. C. Johansson, B. S. L. Collins, M. J. Gaunt, Angew. Chem. 2011, 123, 1108; Angew. Chem. Int. Ed. 2011, 50, 1076;
- 9hZ. Shi, B. Li, X. Wan, J. Cheng, Z. Fang, B. Cao, C. Qin, Y. Wang, Angew. Chem. 2007, 119, 5650; Angew. Chem. Int. Ed. 2007, 46, 5554.
- 10
- 10aN. Umeda, K. Hirano, T. Satoh, N. Shibata, H. Sato, M. Miura, Org. Lett. 2005, 7, 2229;
- 10bS. Miyamura, H. Tsurugi, T. Satoh, M. Miura, J. Organomet. Chem. 2008, 693, 2438;
- 10cT. Vogler, A. Studer, Org. Lett. 2008, 10, 129.
- 11
- 11aK. Ueura, T. Satoh, M. Miura, Org. Lett. 2009, 11, 5198;
- 11bT. Fukutani, K. Hirano, T. Satoh, M. Miura, J. Org. Chem. 2011, 76, 2867.
- 12
- 12aD. Weltin, V. Picard, K. Aupeix, M. Varin, D. Oth, J. Marchal, P. Dufour, P. Bischoff, Int. J. Immunopharmac. 1995, 17, 265;
- 12bT. Harayama, H. Akamatsu, K. Okamura, T. Miyagoe, T. Akiyama, H. Abe, Y. Takeuchi, J. Chem. Soc. Perkin Trans. 1 2001, 523;
- 12cT. Harayama, T. Akiyama, Y. Nakano, K. Shibaike, H. Akamatsu, A. Hori, H. Abe, Y. Takeuchi, Synthesis 2002, 237.
- 13
- 13aG.-W. Wang, T.-T. Yuan, D.-D. Li, Angew. Chem. 2011, 123, 1416; Angew. Chem. Int. Ed. 2011, 50, 1380;
- 13bJ. Karthikeyan, C.-H. Cheng, Angew. Chem. 2011, 123, 10054;
10.1002/ange.201104311 Google ScholarAngew. Chem. Int. Ed. 2011, 50, 9880.
- 14
- 14aK. Parthasarathy, M. Jeganmohan, C.-H. Cheng, Org. Lett. 2008, 10, 325;
- 14bV. S. Thirunavukkarasu, K. Parthasarathy, C.-H. Cheng, Angew. Chem. 2008, 120, 9604;
10.1002/ange.200804153 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 9462;
- 14cV. S. Thirunavukkarasu, K. Parthasarathy, C.-H. Cheng, Chem. Eur. J. 2010, 16, 1436;
- 14dP. Gandeepan, K. Parthasarathy, C.-H. Cheng, J. Am. Chem. Soc. 2010, 132, 8569;
- 14eP. Gandeepan, C.-H. Cheng, J. Am. Chem. Soc. 2012, 134, 5738;
- 14fK. Parthasarathy, N. Senthilkumar, J. Jayakumar, C.-H. Cheng, Org. Lett. 2012, 14, 3478.
- 15
- 15aL. Li, W. W. Brennessel, W. D. Jones, Organometallics 2009, 28, 3492;
- 15bL. Li, W. W. Brennessel, W. D. Jones, J. Am. Chem. Soc. 2008, 130, 12414;
- 15cD. R. Stuart, P. Alsabeh, M. Kuhn, K. Fagnou, J. Am. Chem. Soc. 2010, 132, 18326.
- 16E. M. Simmons, J. F. Hartwig, Angew. Chem. 2012, 124, 3120;
10.1002/ange.201107334 Google ScholarAngew. Chem. Int. Ed. 2012, 51, 3066.