Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture: Primary-Amine-Catalyzed Enantioselective Intramolecular Aldolizations (Angew. Chem. Int. Ed. 40/2008)
- Page: 7565
- First Published: 22 September 2008

Liquorice or celery the catalyst decides! The two enantiomers of celery ketone differ strongly in their scent, and both can be made from the same symmetric diketone precursor by using a new organocatalytic aldol reaction. The enantiogroup-selective intramolecular aldolization of 4-propylheptane-2,6-dione provides either the S enantiomer, which smells of liquorice, or the potent R enantiomer, which determines the celery odor of the racemate. For details, see the Communication by B. List and co-workers on page 7656 ff.
Inside Cover
Inside Cover: Ribosomal Synthesis of Tricyclic Depsipeptides in Bloom-Forming Cyanobacteria (Angew. Chem. Int. Ed. 40/2008)
- Page: 7566
- First Published: 22 September 2008

Cage-like depsipeptides are investigated by C. Hertweck, E. Dittmann, and co-workers in their Communication on page 7756 ff. The cyanobacterial protease inhibitors microviridin B and J are synthesized from ribosomally produced prepeptides that are transformed into tricyclic depsipeptides. The picture shows a microviridin-producing cyanobacteria toxic bloom on a lake surface, together with a schematic representation of ribosomal protein biosynthesis and tricyclic depsipeptide cage structures.
Graphical Abstract
Graphical Abstract: Angew. Chem. Int. Ed. 40/2008
- Pages: 7569-7580
- First Published: 22 September 2008
Corrigendum
Nickel-Mediated Coupling Reactions of Carboryne with Alkenes: A Synthetic Route to Alkenylcarboranes
- Page: 7580
- First Published: 22 September 2008
News
Spotlights on our sister journals: Angew. Chem. Int. Ed. 40/2008
- Pages: 7584-7585
- First Published: 22 September 2008
Medicinal Chemistry: Ley, Seeberger, and Kubinyi Awarded
- Page: 7586
- First Published: 22 September 2008
Book Review
Ferrocenes. Ligands, Materials and Biomolecules. Edited by Petr Štěpnička.
- Page: 7587
- First Published: 22 September 2008
Highlight
Bioinorganic Chemistry
Carboxylate-Bridged Dinuclear Active Sites in Oxygenases: Diiron, Dimanganese, or is Heterodinuclear Better?
- Pages: 7588-7591
- First Published: 22 September 2008
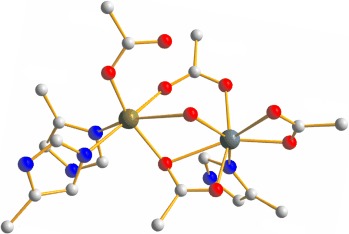
To mix or not to mix: The N-oxygenase AurF of S. thioluteus has an unusual carboxylate-bridged dinuclear active site (see picture; gray C, blue N, red O, green Mn, turquoise Mn/Fe). The similarity with a recently discovered Mn/Fe-oxygenase subunit of the ribonucleotide reductase of C. trachomatis suggests that it might contain both Mn and Fe. The N-oxygenase of S. thioluteus very likely represents the first member of a new family of Mn/Fe-oxygenases.
Minireview
Reaction Pathway Bifurcations
Bifurcations on Potential Energy Surfaces of Organic Reactions
- Pages: 7592-7601
- First Published: 22 September 2008
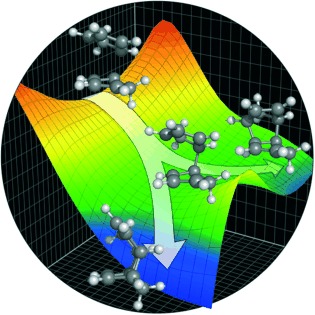
Path of least resistance: Reaction pathway bifurcations have been identified for many complex organic transformations. This type of potential energy surface describes a two-step-no-intermediate reaction mechanism that is neither stepwise nor concerted. Selective formation of one product over another is governed by the potential energy surface shape and resulting dynamic effects, not by transition state energetics.
Review
Biosensors
Semiconductor Quantum Dots for Bioanalysis
- Pages: 7602-7625
- First Published: 22 September 2008
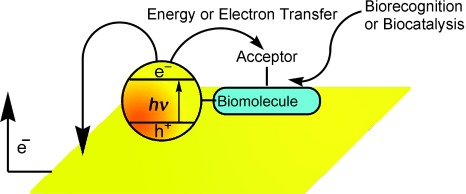
Hybrids of biomolecules and semiconductor quantum dots (QDs) are functional units for the optical, photoelectrochemical, and electrochemical analysis of biorecognition events or biocatalyzed transformations. The size-controlled fluorescence properties of QDs enable multiplexed bioanalysis, and by their coupling to appropriate quencher units, the optical probing of dynamic biocatalytic processes is feasible. The integration of electrodes into biomolecule–QD hybrids allows the measurement of biorecognition processes or transformations by photocurrents.
Communications
Peptide Recognition
Mechanism of Fast Peptide Recognition by SH3 Domains†
- Pages: 7626-7630
- First Published: 22 September 2008
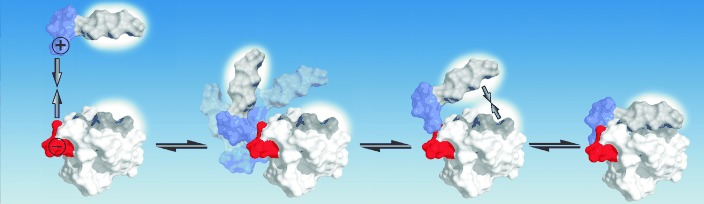
The complete pathway of the association of a proline-rich motif to an SH3 domain was probed by molecular dynamics simulations. The results indicate a bimodal binding mechanism, in which nature uses the reduction in dimensionality and hydrophobic dewetting as tools to find a simple solution to a seemingly complicated binding process.
Ionic Liquids (1)
Europium-Based Ionic Liquids as Luminescent Soft Materials†
- Pages: 7631-7634
- First Published: 22 September 2008
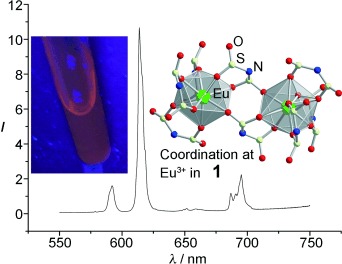
Low melting, highly luminescent: [C3mim][Eu(Tf2N)4] (1), [C4mim][Eu(Tf2N)4], and [C4mpyr]2[Eu(Tf2N)5] are the first lanthanide ionic liquids that do not need stabilization of the liquid state by neutral coligands. They show excellent photophysical properties, such as long lifetimes of luminescence at large EuIII concentration, small line width, and high color purity (see emission spectrum of 1 and photograph of a sample under UV light).
Ionic Liquids (2)
Dysprosium Room-Temperature Ionic Liquids with Strong Luminescence and Response to Magnetic Fields†
- Pages: 7635-7638
- First Published: 22 September 2008
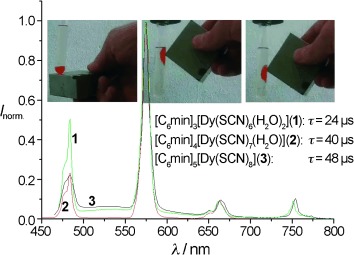
Attractive liquids: Dysprosium-based ionic liquids 1–3 show the highest response to external magnetic fields to date, allowing magnetic manipulation of the liquid (see pictures). At the same time, the liquids have excellent photophysical properties, such as long luminescence decay times τ and high color purity. The figure shows emission spectra of the ionic liquids.
Anisotropic Nanoparticles
Imidazolium-Based Ionic Liquids as Efficient Shape-Regulating Solvents for the Synthesis of Gold Nanorods†
- Pages: 7639-7643
- First Published: 22 September 2008
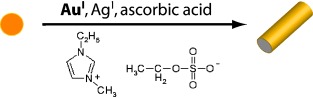
Shape control: Gold nanorods are synthesized in high yields in the absence of shape-regulating agents in 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][ES]). A two-step seeded-growth process is used that capitalizes on the binding affinity of imidazolium cations to gold crystal facets, the stabilization of AuI in [EMIM][ES], and the reduction of particle growth rates that is facilitated by weak reducing agents.
Light-Regulated Enzyme Activity
Regulation of Human Carbonic Anhydrase I (hCAI) Activity by Using a Photochromic Inhibitor†
- Pages: 7644-7647
- First Published: 22 September 2008
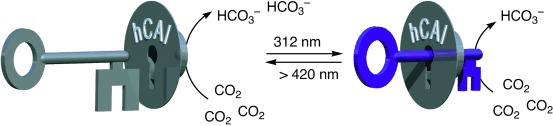
Photoswitchable: The activity of a dithienylethene-based human carbonic anhydrase inhibitor (hCAI) can be regulated using light. Converting the flexible, ring-open isomer of the photoswitch into the rigid, ring-closed isomer using UV light reduces the inhibition and increases the activity of hCAI by 50-fold. The inhibitor can be turned back on by using visible light, which has many advantages in biological applications.
Functionalized Indium Reagents
Preparation of Aryl and Heteroaryl Indium(III) Reagents by the Direct Insertion of Indium in the Presence of LiCl†
- Pages: 7648-7651
- First Published: 22 September 2008
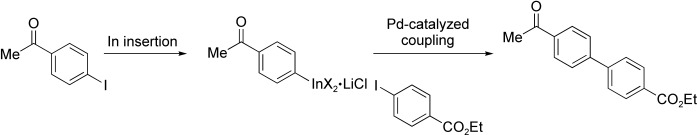
Sensitive functional groups, including ketone, aldehyde, and ester groups, may be present in aryl indium reagents prepared in good to excellent yields by the treatment of aryl and heteroaryl iodides with indium powder in the presence of LiCl (see example). These functionalized organoindium(III) reagents readily undergo Pd-catalyzed cross-coupling with functionalized aryl iodides, including those containing NH or OH groups.
Allylic Substitution
Iridium-Catalyzed Asymmetric Allylic Substitutions—Very High Regioselectivity and Air Stability with a Catalyst Derived from Dibenzo[a,e]cyclooctatetraene and a Phosphoramidite†
- Pages: 7652-7655
- First Published: 22 September 2008
![Iridium-Catalyzed Asymmetric Allylic Substitutions—Very High Regioselectivity and Air Stability with a Catalyst Derived from Dibenzo[a,e]cyclooctatetraene and a Phosphoramidite](/cms/asset/2595aecc-1748-433d-8922-1ba1dc6ca84d/mcontent.jpg)
A final tweak: A new phosphoramidite iridium catalyst (see scheme) allows allylic substitutions to be run with a higher degree of regioselectivity than with other iridium catalysts and under aerobic conditions. Mechanistic aspects, in particular, the reversibility of the catalyst formation by CH activation, are also presented. 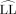 =dibenzocyclooctatetraene.
=dibenzocyclooctatetraene.
Organocatalysis
Primary-Amine-Catalyzed Enantioselective Intramolecular Aldolizations†
- Pages: 7656-7658
- First Published: 22 September 2008
Aldol cyclodehydration of 4-substituted-2,6-heptanediones leads to enantiomerically enriched 5-substituted-3-methyl-2-cyclohexene-1-ones, which serve as perfume ingredients and valuable synthetic building blocks. Primary amines derived from cinchona alkaloids in combination with acetic acid are efficient catalysts for this transformation, which deliver both enantiomers of the celery ketone.
Lewis Superacids
Simple Access to the Non-Oxidizing Lewis Superacid PhF→Al(ORF)3 (RF=C(CF3)3)
- Pages: 7659-7663
- First Published: 22 September 2008
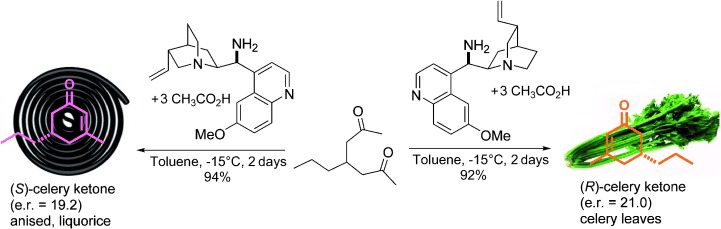
Lewis superacidity? In analogy to Brønsted superacids, Lewis superacids can be defined as Lewis acids that are stronger than the strongest conventional and commercially employed Lewis acid SbF5. The fluorobenzene complex PhF→Al(ORF)3 (RF=C(CF3)3) qualifies as an easily accessible, non-oxidizing and stable Lewis acid that conforms with our Lewis superacidity criterion.
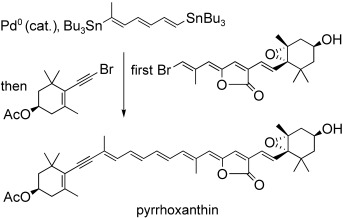
Carotenoids
Total Synthesis of Naturally Configured Pyrrhoxanthin, a Carotenoid Butenolide from Plankton†
- Pages: 7664-7668
- First Published: 22 September 2008
Synthetic Methods
C1-Symmetric Monosubstituted Chiral Diene Ligands in Asymmetric Rhodium-Catalyzed 1,4-Addition Reactions†
- Pages: 7669-7672
- First Published: 22 September 2008
Synthesis of Biaryl Compounds through Three-Component Assembly: Ambidentate Effect of the tert-Butyldimethylsilyl Group for Regioselective Diels–Alder and Hiyama Coupling Reactions†
- Pages: 7673-7676
- First Published: 22 September 2008
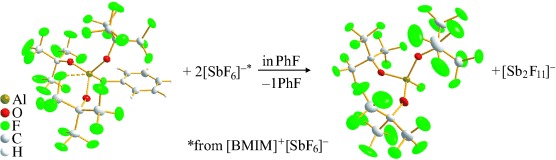
Two for the price of one: A method has been developed for the regiocontrolled synthesis of multisubstituted biaryl derivatives. This protocol involves the use of the tert-butyldimethylsilyl (TBDMS) group to direct the regioselective Diels–Alder reaction of a 3-TBDMS-benzyne with a furan derivative and a subsequent Hiyama cross-coupling reaction of the TBDMS group with aryl iodides (see scheme).
Quantum Dots
Synthetic Scheme for High-Quality InAs Nanocrystals Based on Self-Focusing and One-Pot Synthesis of InAs-Based Core–Shell Nanocrystals†
- Pages: 7677-7680
- First Published: 22 September 2008
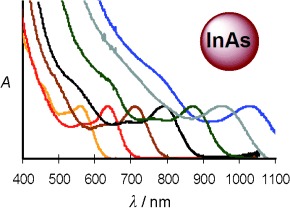
Paint by number: High-quality InAs quantum dots (QDs) for near-IR fluorescence (see picture) were synthesized by exploiting interparticle diffusion and self-focusing instead of using the traditional focusing of size distribution. InAs/CdSe core–shell QDs were grown and analyzed by a technique combining TEM and elemental analysis to determine the sizes and molar extinction coefficients of the extremely small InAs particles.
Nanostructures
Spontaneous Growth of Highly Conductive Two-Dimensional Single-Crystalline TiSi2 Nanonets†
- Pages: 7681-7684
- First Published: 22 September 2008

Net result: Two-dimensional nanonets form spontaneously in a chemical vapor deposition reaction. All beams in the nanonet are single-crystalline nanobelts that are connected by 90° joints. The nanonets are roughly 15 nm thick and a few micrometers long and wide (see pictures for three images at different tilting angles), and they assume a C49 TiSi2 structure.
Magnetic Nanocups
Hollow Spheres to Nanocups: Tuning the Morphology and Magnetic Properties of Single-Crystalline α-Fe2O3 Nanostructures†
- Pages: 7685-7688
- First Published: 22 September 2008
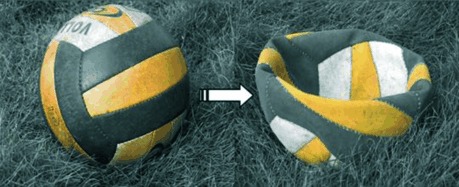
A bottom-up approach has been used to tune the morphology of single-crystalline hematite from hollow spheres to nanocups. The mechanism involves the formation of nanocups through buckling of the spheres, similar to a deflated ball (see picture). As the shape changes, there is a drastic change in magnetic properties.
Oxidative Methane Coupling
Rate and Selectivity Enhancements Mediated by OH Radicals in the Oxidative Coupling of Methane Catalyzed by Mn/Na2WO4/SiO2†
- Pages: 7689-7693
- First Published: 22 September 2008
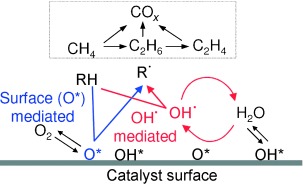
Radically Improved: OH radicals formed by quasi-equilibrated steps on oxide surfaces introduce homogeneous pathways that lead to higher rates and C2 yields in oxidative methane coupling relative to those attained by CH4 activation with chemisorbed oxygen (see picture; O*: dissociated oxygen atom; R: abstractor). The reactivity of OH. leads to a weaker influence of the CH bond energies on the relative rates of H abstraction from CH4, C2H6, and C2H4.
Olefin Hydroarylation
Intermolecular Hydroarylation of Unactivated Olefins Catalyzed by Homogeneous Platinum Complexes†
- Pages: 7694-7696
- First Published: 22 September 2008
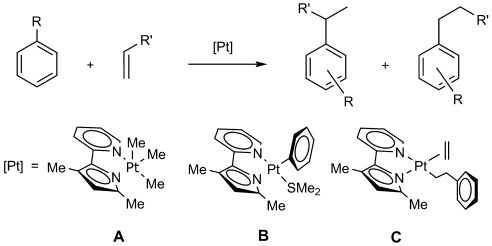
Designing catalysts: The five-coordinate platinum(IV) complex A and the platinum(II) trans complex B act as precatalysts for the hydroarylation of unactivated olefins. The catalytic cycle features an aryl–olefin insertion at PtII and a CH bond activation of the arene solvent as key steps. The PtII cis complex C has been observed in hydroarylation reactions of ethylene with benzene.
Bioinorganic Chemistry
Bioinspired Dismutation of Chlorite to Dioxygen and Chloride Catalyzed by a Water-Soluble Iron Porphyrin†
- Pages: 7697-7700
- First Published: 22 September 2008
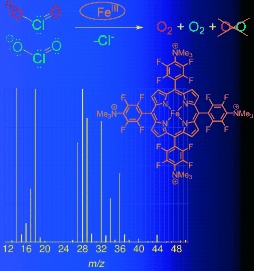
Cleaning up chlorite: A water-soluble iron porphyrin catalyzes the dismutation of chlorite to dioxygen and chloride. Labeling experiments demonstrate a novel mechanism for OO bond formation. These mechanistic insights should aid in the design of catalysts for remediation of oxychlorine contaminants.
Hydrosilylation
Agostic NSiH⋅⋅⋅Mo Complexes: From Curiosity to Catalysis†
- Pages: 7701-7704
- First Published: 22 September 2008
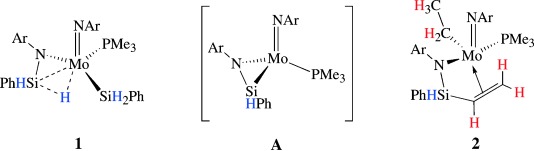
An uncommon catalyst: The β-agostic NSiH⋅⋅⋅M complex 1 (Ar=2,6-diisopropylphenyl) catalyzes a variety of hydrosilylation reactions. Stoichiometric reactions of 1 with unsaturated compounds proceed via the silanimine intermediate A and, in the case of olefins or nitriles, give products of SiC coupling, such as 2.
Molecular Codes
Tunable Molecular Assembly Codes Direct Reaction Pathways†
- Pages: 7705-7709
- First Published: 22 September 2008
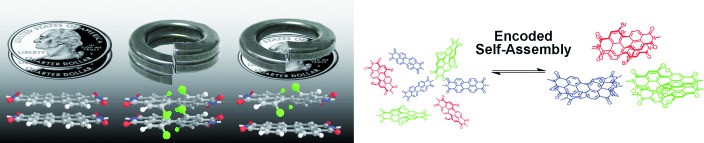
In the twist: The extent of twisting from planarity in a series of perylenetetracarboxylic diimides (PDIs) modulates the attractive π–π stacking force, revealing an array of inherent molecular recognition codes. Such coded self-assembly directs specific reaction pathways so that a mixture of reactive monomers with different codes results in identical products as when the reactions were carried out in separate flasks (see picture).
Organocatalysis
Asymmetric Aza-Michael Reactions of α,β-Unsaturated Ketones with Bifunctional Organic Catalysts†
- Pages: 7710-7713
- First Published: 22 September 2008
Synergy makes it possible: Bifunctional cinchona alkaloids are found to promote the first highly enantioselective organocatalytic aza-Michael reactions with α,β-unsaturated ketones. In addition to utilizing practical catalysts, readily accessible substrates, and commercially available reagents, this reaction affords a synthetically valuable scope that is complimentary to existing methods catalyzed by chiral metal complexes.
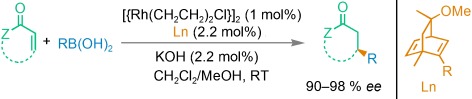
Asymmetric Catalysis
Modularly Designed Organocatalytic Assemblies for Direct Nitro-Michael Addition Reactions†
- Pages: 7714-7717
- First Published: 22 September 2008

It's so simple! Organocatalysts formed through the self-assembly of simple α-amino acids and alkaloid thiourea derivatives (see scheme) are used as highly efficient catalysts for the direct nitro-Michael addition of ketones and nitroalkenes, affording excellent ee values up to 99 %. Enantioselectivity may be tuned by changing components of the self-assembled catalyst.
Grignard Reagents
Enantioselective Cu-Catalyzed 1,4-Addition of Grignard Reagents to Cyclohexenone Using Taddol-Derived Phosphine–Phosphite Ligands and 2-Methyl-THF as a Solvent†
- Pages: 7718-7721
- First Published: 22 September 2008
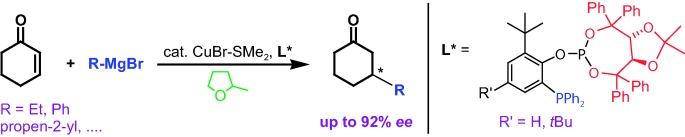
A small library of modular P,P ligands was screened and a potent Cu-based catalyst system was identified for highly enantioselective 1,4-additions to cyclohexenone with an unprecedented broad spectrum of Grignard reagents. Surprisingly, the highest selectivities were achieved in most cases in 2-methyl-THF, a “green” solvent underestimated so far.
Nanoscale Metal–Organic Frameworks
Surfactant-Assisted Synthesis of Nanoscale Gadolinium Metal–Organic Frameworks for Potential Multimodal Imaging†
- Pages: 7722-7725
- First Published: 22 September 2008
Less basic: By controlling the pH value of the reaction medium, two different gadolinium-containing nanoscale metal–organic frameworks (NMOFs) based on the same building blocks can be synthesized (see picture). The NMOFs carry a large payload of Gd3+ centers and are efficient contrast agents for T2-weighted magnetic resonance imaging. The NMOFs are highly luminescent when doped with other lanthanide ions such as Eu and Tb.
Supramolecular Surface Chemistry
Trimodular Engineering of Linear Supramolecular Miniatures on Ag(111) Surfaces Controlled by Complementary Triple Hydrogen Bonds†
- Pages: 7726-7730
- First Published: 22 September 2008
Simultaneous three-component assembly on surfaces mediated by triple H-bonding interactions leading to the formation of linear supramolecular miniatures has been studied on Ag(111) surfaces by STM. In particular, the complementary assembly of two linear modules (see picture, blue and green) and an anthracenyl-capped molecular stopper (red) led to the formation of discrete linear oligomeric, pentameric, and trimeric nanoassemblies.
Electrochemiluminescence
Tuning of Electrogenerated Silole Chemiluminescence†
- Pages: 7731-7735
- First Published: 22 September 2008
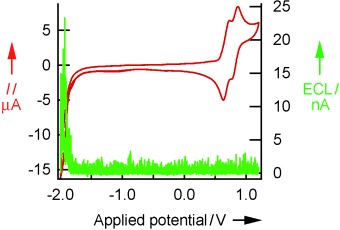
Electrochemical tuning and structural modification of thiophene-substituted siloles led to silole chromophores with efficient and stable electrochemiluminescence (ECL). By extending silole π conjugation with thiophene units and by constraining the applied potential range, stable radical cations favorable for ECL emission were generated (see graph for an example).
Pharmacophore Mapping
Crystallography-Independent Determination of Ligand Binding Modes†
- Pages: 7736-7740
- First Published: 22 September 2008
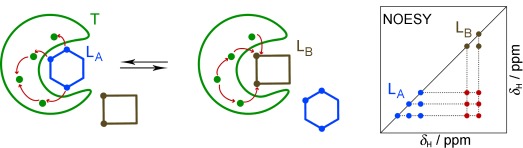
Pass the spin: The internuclear NOE interactions for pharmacophore mapping (INPHARMA) can be used to derive the structure of receptor/ligand complexes for low-affinity lead compounds identified in the early stages of drug discovery. The relative binding mode and, in favorable cases, the absolute binding mode of pairs of competitive low-affinity ligands can be identified with INPHARMA. This is demonstrated for a system comprising protein kinase A (T in the schematic representation) and two activity inhibitors with known structures (LA and LB).
Hydrogen Storage
A Comparison of the H2 Sorption Capacities of Isostructural Metal–Organic Frameworks With and Without Accessible Metal Sites: [{Zn2(abtc)(dmf)2}3] and [{Cu2(abtc)(dmf)2}3] versus [{Cu2(abtc)}3]†
- Pages: 7741-7745
- First Published: 22 September 2008
![A Comparison of the H2 Sorption Capacities of Isostructural Metal–Organic Frameworks With and Without Accessible Metal Sites: [{Zn2(abtc)(dmf)2}3] and [{Cu2(abtc)(dmf)2}3] versus [{Cu2(abtc)}3]](/cms/asset/76bba14b-2555-47fd-87e3-23adcfd8f91c/mcontent.jpg)
The isostructural metal–organic frameworks [{Zn2(abtc)(dmf)2}3] (1 a), [{Cu2(abtc)(dmf)2}3] (2 a), and [{Cu2(abtc)}3] (2 b; H4abtc=1,1′-azobenzene-3,3′,5,5′-tetracarboxylic acid) have high sorption capacities for H2, N2, CO2, and CH4. Solid 2 b, which has accessible metal sites (AMSs), has a higher H2 adsorption capacity than 1 a or 2 a (see picture; T=77 K), neither of which have AMSs, because of its lower molecular weight and greater isosteric heat of H2 adsorption.
Density Functional Calculations
Sources of Error in DFT Computations of CC Bond Formation Thermochemistries: π→σ Transformations and Error Cancellation by DFT Methods†
- Pages: 7746-7749
- First Published: 22 September 2008
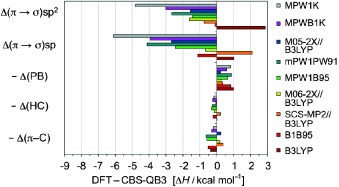
Alarming: Multiple sources of errors in DFT energetics of CC bond-forming reactions were investigated by evaluating structural transformations in Diels–Alder reactions: conversion of π into σ bonds and changes in conjugation, hyperconjugation, and branching interactions. A startling overestimation of the π to σ bond conversion is found with most methods, a central problem to all reactions involving addition of π bonds (electrocyclic processes, ene, aldol).
Photomagnetic Nanocomposites
Controlled Synthesis of Photomagnetic Nanoparticles of a Prussian Blue Analogue in a Silica Xerogel†
- Pages: 7750-7752
- First Published: 22 September 2008

Controlled precipitation: Silica can control the precipitation of a CoFe Prussian Blue analogue (PBA) by reversibly protecting the CoII ions. The precursors are introduced into a silica sol and precipitation of the PBA is triggered by acidification after condensation of the silica network (see picture). This original method provides a homogeneous nanocomposite with important photomagnetic properties.
Synchronized Nano-Oscillators
Diffusively Coupled Chemical Oscillators in a Microfluidic Assembly†
- Pages: 7753-7755
- First Published: 22 September 2008
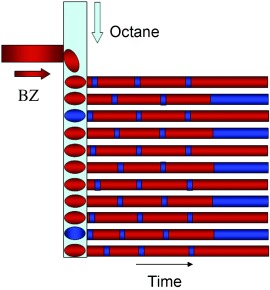
Fascillating: Inhibitory coupling by bromine among nanoliter aqueous droplets containing the Belousov–Zhabotinsky (BZ) reaction solution produces anti-phase oscillations and stationary Turing patterns (see picture; reduced catalyst in red, oxidized catalyst in blue). By choosing the fundamental oscillator and the scavenger added to the connecting medium, it should be possible to construct systems with controllable degrees of inhibitory or excitatory coupling.
Natural Products
Ribosomal Synthesis of Tricyclic Depsipeptides in Bloom-Forming Cyanobacteria†
- Pages: 7756-7759
- First Published: 22 September 2008
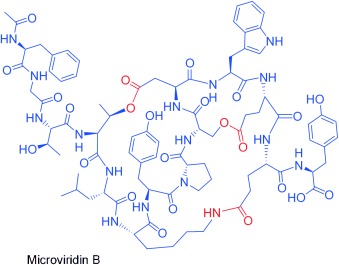
Decoding biosynthesis: Cage-like depsipeptides have been investigated by a combination of genetic and chemical analyses. Heterologous expression and MALDI-PSD studies reveal that the cyanobacterial protease inhibitors microviridin B and J are synthesized from ribosomally produced prepeptides that are transformed into tricyclic depsipeptides by ATP grasp ligases and processed by a transporter peptidase.
Molecule-Based Magnets
An Electron-Transfer Ferromagnet with Tc=107 K Based on a Three-Dimensional [Ru2]2/TCNQ System†
- Pages: 7760-7763
- First Published: 22 September 2008
![An Electron-Transfer Ferromagnet with Tc=107 K Based on a Three-Dimensional [Ru2]2/TCNQ System](/cms/asset/1870b7cb-a62b-48d5-8119-46dc948cb742/mcontent.jpg)
Electron-transfer makes a magnet: 2:1 assembly of [Ru2II,IIL4] paddlewheel complexes and BDTA-TCNQ yielded a new structural type of electron-transfer system with a three-dimensional network structure which exhibits a long-range ferromagnetic transition at Tc=107 K (see depicted χ vs T curve and inset view of structure along the a axis). L=m-fluorobenzoate, BTDA-TCNQ=bis(1,2,5-thiadiazolo)tetracyanoquinodimethane.
Protein Splicing
Activation of Protein Splicing by Protease- or Light-Triggered O to N Acyl Migration†
- Pages: 7764-7767
- First Published: 22 September 2008
Protein splicing under control: Introduction of an O-acyl isomer of a peptide bond into the naturally split Ssp DnaE intein prevents protein trans splicing, which can be recovered upon triggering an O to N acyl shift by deprotection with protease or light (see scheme). This system allows the splicing of a bacterial toxin to be controlled in response to protease activity.
Preview
Preview: Angew. Chem. Int. Ed. 41/2008
- Page: 7771
- First Published: 22 September 2008