Enantioselective Cu-Catalyzed 1,4-Addition of Grignard Reagents to Cyclohexenone Using Taddol-Derived Phosphine–Phosphite Ligands and 2-Methyl-THF as a Solvent†
Tobias Robert Dipl.-Chem.
Institut für Organische Chemie, Universität zu Köln, Greinstrasse 4, 50939 Köln (Germany), Fax: (+49) 221-470-3064
Search for more papers by this authorJanna Velder Dr.
Institut für Organische Chemie, Universität zu Köln, Greinstrasse 4, 50939 Köln (Germany), Fax: (+49) 221-470-3064
Search for more papers by this authorHans-Günther Schmalz Prof. Dr.
Institut für Organische Chemie, Universität zu Köln, Greinstrasse 4, 50939 Köln (Germany), Fax: (+49) 221-470-3064
Search for more papers by this authorTobias Robert Dipl.-Chem.
Institut für Organische Chemie, Universität zu Köln, Greinstrasse 4, 50939 Köln (Germany), Fax: (+49) 221-470-3064
Search for more papers by this authorJanna Velder Dr.
Institut für Organische Chemie, Universität zu Köln, Greinstrasse 4, 50939 Köln (Germany), Fax: (+49) 221-470-3064
Search for more papers by this authorHans-Günther Schmalz Prof. Dr.
Institut für Organische Chemie, Universität zu Köln, Greinstrasse 4, 50939 Köln (Germany), Fax: (+49) 221-470-3064
Search for more papers by this authorThis work was carried out in the context of Cost D40 and supported by the European Commission (Ligbank), the Chemetall GmbH, and the Fonds der Chemischen Industrie.
Graphical Abstract
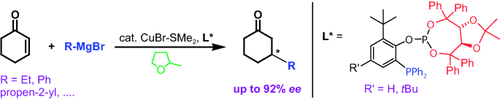
A small library of modular P,P ligands was screened and a potent Cu-based catalyst system was identified for highly enantioselective 1,4-additions to cyclohexenone with an unprecedented broad spectrum of Grignard reagents. Surprisingly, the highest selectivities were achieved in most cases in 2-methyl-THF, a “green” solvent underestimated so far.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z803247_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Reviews:
- 1aP. Perlmutter, Tetrahedron Organic Chemistry Series 9; Pergamon, Oxford, UK, 1992;
- 1bV. J. Lee in Comprehensive Organic Synthesis, Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, pp. 69–137;
10.1016/B978-0-08-052349-1.00091-3 Google Scholar
- 1cV. J. Lee in Comprehensive Organic Synthesis, Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, pp. 139–168;
- 1dJ. A. Kozlowski in Comprehensive Organic Synthesis, Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, pp. 169–198.
10.1016/B978-0-08-052349-1.00093-7 Google Scholar
- 2Reviews:
- 2aH.-G. Schmalz in Comprehensive Organic Synthesis, Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, pp. 199–236;
10.1016/B978-0-08-052349-1.00094-9 Google Scholar
- 2bB. E. Rossiter, N. M. Swingle, Chem. Rev. 1992, 92, 771–806.
- 3
- 3aN. Krause, A. Hoffmann-Röder, Synthesis 2001, 171–196;
- 3bJ. Christoffers, G. Koripelly, A. Rosiak, M. Rössle, Synthesis 2007, 1279–1300;
- 3cK. Tomioka, Y. Nagaoka in Comprehensive Asymmetric Catalysis, Vol. III (Eds.: ), Springer, Berlin, 1999, pp. 1105–1120.
10.1007/978-3-642-58571-5_5 Google Scholar
- 4
- 4aReview: F. López, A. J. Minaard, B. L. Feringa, Acc. Chem. Res. 2007, 40, 179–188; for key publications in this field, see:
- 4bK.-H. Ahn, R. B. Klassen, S. J. Lippard, Organometallics 1990, 9, 3178–3181;
- 4cD. M. Knotter, D. M. Grove, W. J. J. Smeets, A. L. Spek, G. van Koten, J. Am. Chem. Soc. 1992, 114, 3400–3410
- 4dD. Spescha, G. Rihs, Helv. Chim. Acta 1993, 76, 1219–1230;
- 4eQ.-L. Zhou, A. Pfaltz, Tetrahedron 1994, 50, 4467–4478;
- 4fA. L. Braga, S. J. N. Silva, D. S. Lüdtke, R. L. Drekener, C. C. Silveira, J. B. T. Rocha, L. A. Wessjohann, Tetrahedron Lett. 2002, 43, 7329–7331;
- 4gD. Seebach, G. Jaeschke, A. Pichota, L. Audergon, Helv. Chim. Acta 1997, 80, 2515–2519;
- 4hM. Kanai, Y. Nakagawa, K. Tomioka, Tetrahedron 1999, 55, 3843–3854;
- 4iE. L. Stangeland, T. Sammakia, Tetrahedron 1997, 53, 16503–16510.
- 5T. Ireland, G. Grossheimann, C. Wieser-Jeunesse, P. Knochel, Angew. Chem. 1999, 111, 3397–3400;
10.1002/(SICI)1521-3757(19991102)111:21<3397::AID-ANGE3397>3.0.CO;2-R Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3212–3215.10.1002/(SICI)1521-3773(19991102)38:21<3212::AID-ANIE3212>3.0.CO;2-9 CAS PubMed Web of Science® Google Scholar
- 6A. Togni, C. Breutel, A. Schnyder, F. Spindler, H. Landert, A. Tijani, J. Am. Chem. Soc. 1994, 116, 4062–4066.
- 7B. L. Feringa, R. Badorrey, D. Peña, S. R. Harutyunyan, A. J. Minaard, Proc. Natl. Acad. Sci. USA 2004, 101, 5834–5838.
- 8
- 8aF. López, S. R. Harutyunyan, A. J. Minaard, B. L. Feringa, J. Am. Chem. Soc. 2004, 126, 12784–12785;
- 8bF. López, S. R. Harutyunyan, A. Meetsma, A. J. Minaard, B. L. Feringa, Angew. Chem. 2005, 117, 2812–2816; Angew. Chem. Int. Ed. 2005, 44, 2752–2756;
- 8cS. R. Harutyunyan, F. López, W. R. Browne, A. Correa, D. Peña, R. Badorrey, A. Meetsma, A. J. Minaard, B. L. Feringa, J. Am. Chem. Soc. 2006, 128, 9103–9118;
- 8dR. Des Mazery, M. Pullez, F. López, S. R. Harutyunyan, A. J. Minaard, B. L. Feringa, J. Am. Chem. Soc. 2005, 127, 9966–9967;
- 8eS.-Y. Wang, S.-J. Ji, T.-P. Loh, J. Am. Chem. Soc. 2007, 129, 276–277;
- 8fT.-K. Lum, S.-Y. Wang, T.-P. Loh, Org. Lett. 2008, 10, 761–764.
- 9
- 9aJ. Velder, T. Robert, I. Weidner, J.-M. Neudörfl, J. Lex, H.-G. Schmalz, Adv. Synth. Catal. 2008, 350, 1309–1315;
- 9bF. Blume, S. Zemolka, T. Fey, R. Kranich, H.-G. Schmalz, Adv. Synth. Catal. 2002, 344, 868–883;
- 9cR. Kranich, K. Eis, O. Geis, S. Mühle, J. W. Bats, H.-G. Schmalz, Chem. Eur. J. 2000, 6, 2874–2894.
10.1002/1521-3765(20000804)6:15<2874::AID-CHEM2874>3.0.CO;2-1 CAS PubMed Web of Science® Google Scholar
- 10For the application of ligands of type 7 in the highly enantioselective Rh-catalyzed hydroboration of styrenes, see Ref. [9b] as well as: S. Werle, T. Fey, J. M. Neudörfl, H.-G. Schmalz, Org. Lett. 2007, 9, 3555–3558; Erratum: S. Werle, T. Fey, J. M. Neudörfl, H.-G. Schmalz, Org. Lett. 2007, 9, 4085.
- 11For a recent review, see:
- 11aJ. M. Brunel, Chem. Rev. 2005, 105, 857–898; Erratum: J. M. Brunel, Chem. Rev. 2005, 105, 857–898; for an update, see
- 11bJ. M. Brunel, Chem. Rev. 2007, 107, 1–45.
- 12
- 12aD. Seebach, A. K. Beck, R. Imwinkelried, S. Roggo, A. Wonnacott, Helv. Chim. Acta 1987, 70, 954. For a review, see:
- 12bD. Seebach, A. K. Beck, A. Heckel, Angew. Chem. 2001, 113, 96–142;
10.1002/1521-3757(20010105)113:1<96::AID-ANGE96>3.0.CO;2-B Google ScholarAngew. Chem. Int. Ed. 2001, 40, 92–138.
- 13In a control experiment under exactly the same conditions, ligand 1 (Taniaphos) afforded the product (4, R=Et) with 92 % ee, which is in acceptable agreement to the results of Feringa and coworkers (Ref. [7]) who reported 96 % ee with a slightly higher catalyst loading.
- 14For reviews, see:
- 14aE. L. Eliel, S. H. Wilen, L. N. Mander in Stereochemistry of Carbon Compounds, VCH, New York, 1994, pp. 1007–1043;
- 14bD. N. Kirk, Tetrahedron 1986, 42, 777–818;
- 14cJ. K. Gawronski, Tetrahedron 1982, 38, 3–26;
- 14dR. D. Burnett, D. N. Kirk, J. Chem. Soc. Perkin Trans. 1 1981, 1460–1468.
- 15Yields (obtained on a 9 mmol scale) are in the same range as reported by Feringa et al. (Ref. [7]); they are sometimes impaired through the formation of by-products resulting from aldol-type processes.
- 16For asymmetric Cu-catalyzed 1,4-additions of Grignard reagents to 3-alkylcyclohexenones using chiral N-heterocyclic carbene ligands, see:
- 16aD. Martin, S. Kehrli, M. d'Augustin, H. Clavier, M. Mauduit, A. Alexakis, J. Am. Chem. Soc. 2006, 128, 8416–8417;
- 16bY. Matsumoto, K.-i. Yamada, K. Tomioka, J. Org. Chem. 2008, 73, 4578–4581.
- 17To the best of our knowledge, only one example for the use of a chiral phosphine–phosphite ligand in the Cu-catalyzed asymmetric conjugate addition (of Et2Zn and Et3Al) has been reported; see: M. Diéguez, S. Deerenberg, O. Pàmies, C. Claver, P. W. N. M. van Leeuwen, P. C. J. Kamer, Tetrahedron: Asymmetry 2000, 11, 3161–3166.
- 18D. F. Aycock, Org. Process Res. Dev. 2007, 11, 156–159.