Sources of Error in DFT Computations of CC Bond Formation Thermochemistries: π→σ Transformations and Error Cancellation by DFT Methods†
Susan N. Pieniazek Dr.
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569 (USA), Fax: (+1) 310-206-1843
Search for more papers by this authorFernando R. Clemente Dr.
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569 (USA), Fax: (+1) 310-206-1843
Search for more papers by this authorKendall N. Houk Prof. Dr.
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569 (USA), Fax: (+1) 310-206-1843
Search for more papers by this authorSusan N. Pieniazek Dr.
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569 (USA), Fax: (+1) 310-206-1843
Search for more papers by this authorFernando R. Clemente Dr.
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569 (USA), Fax: (+1) 310-206-1843
Search for more papers by this authorKendall N. Houk Prof. Dr.
Department of Chemistry and Biochemistry, University of California, Los Angeles, CA 90095-1569 (USA), Fax: (+1) 310-206-1843
Search for more papers by this authorWe are grateful to the National Science Foundation and the Partnerships for Advanced Computational Infrastructure (PACI) for the financial support of this research. The computations were performed on the UCLA Academic Technology Services (ATS) Hoffman Beowulf cluster.
Graphical Abstract
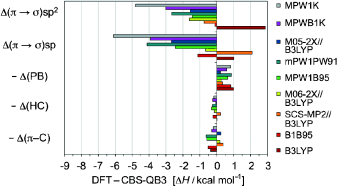
Alarming: Multiple sources of errors in DFT energetics of CC bond-forming reactions were investigated by evaluating structural transformations in Diels–Alder reactions: conversion of π into σ bonds and changes in conjugation, hyperconjugation, and branching interactions. A startling overestimation of the π to σ bond conversion is found with most methods, a central problem to all reactions involving addition of π bonds (electrocyclic processes, ene, aldol).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z801843_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aW. Koch, M. C. Holthausen, A Chemist's Guide to Density Functional Theory, 2nd ed., Wiley-VCH, Weinheim, 2001;
10.1002/3527600043 Google Scholar
- 1bA. Chatterjee, Int. J. Mol. Sci. 2002, 3, 234–236.
- 2
- 2aA. D. Becke, J. Chem. Phys. 1993, 98, 5648–5652;
- 2bP. J. Stephens, F. J. Devlin, C. F. Chablowski, M. J. Frisch, J. Phys. Chem. 1994, 98, 11623–11627;
- 2cC. Lee, W. Yang, R. G. Parr, Phys. Rev. B 1988, 37, 785–789.
- 3
- 3aL. A. Curtiss, K. Raghavachari, P. C. Redfern, J. A. Pople, J. Chem. Phys. 2000, 112, 7374–7383;
- 3bJ. Cioslowski, M. Schimeczek, G. Liu, V. Stoyanov, J. Chem. Phys. 2000, 113, 9377–9389;
- 3cP. Winget, T. Clark, J. Comput. Chem. 2004, 25, 725–733.
- 4T. P. M. Goumans, A. W. Ehlers, K. Lammertsma, E.-U. Würthwein, S. Grimme, Chem. Eur. J. 2004, 10, 6468–6475.
- 5P. R. Schreiner, Angew. Chem. 2007, 119, 4295–4297; Angew. Chem. Int. Ed. 2007, 46, 4217–4219.
- 6P. C. Redfern, P. Zapol, L. A. Curtiss, K. Raghavachari, J. Phys. Chem. A 2000, 104, 5850–5854.
- 7C. E. Check, T. M. Gilbert, J. Org. Chem. 2005, 70, 9828–9834.
- 8
- 8aA. G. Leach, E. Goldstein, K. N. Houk, J. Am. Chem. Soc. 2003, 125, 8330–8339;
- 8bS. M. Bachrach, J. C. Gilbert, J. Org. Chem. 2004, 69, 6357–6364.
- 9
- 9aS. N. Pieniazek, K. N. Houk, Angew. Chem. 2006, 118, 1470–1473;
10.1002/ange.200502677 Google ScholarAngew. Chem. Int. Ed. 2006, 45, 1442–1445;
- 9bA. Padwa, K. R. Crawford, C. S. Straub, S. N. Pieniazek, K. N. Houk, J. Org. Chem. 2006, 71, 5432–5439.
- 10S. Grimme, M. Steinmetz, M. Korth, J. Org. Chem. 2007, 72, 2118–2126.
- 11S. Grimme, Angew. Chem. 2006, 118, 4571–4575; Angew. Chem. Int. Ed. 2006, 45, 4460–4464.
- 12
- 12aH. L. Woodcock, H. F. Schaefer III, P. R. Schreiner, J. Phys. Chem. A 2002, 106, 11923–11931;
- 12bY. Zhao, D. G. Truhlar, J. Phys. Chem. A 2006, 110, 10478–10486;
- 12cY. Zhao, D. G. Truhlar, Org. Lett. 2006, 8, 5753–5755.
- 13
- 13aM. D. Wodrich, C. Corminboeuf, P. von R. Schleyer, Org. Lett. 2006, 8, 3631–3634;
- 13bM. D. Wodrich, C. S. Wannere, Y. Mo, P. D. Jarowski, K. N. Houk, P. von R. Schleyer, Chem. Eur. J. 2007, 13, 7731–7744.
- 14
- 14aA. de Meijere, C.-H. Lee, M. A. Kuznetsov, D. V. Gusev, S. I. Kozhushkov, A. A. Fokin, P. R. Schreiner, Chem. Eur. J. 2005, 11, 6175–6184;
- 14bP. R. Schreiner, A. A. Fokin, R. A. Pascal, A. de Meijere, Org. Lett. 2006, 8, 3635–3638;
- 14cM. D. Wodrich, C. Corminboeuf, P. R. Schreiner, A. A. Fokin, P. von R. Schleyer, Org. Lett. 2007, 9, 1851–1854.
- 15
- 15aAll calculations were performed with the Gaussian 03 suite of programs: M. J. Frisch et al. Gaussian 03, revision C.02; Gaussian Inc.: Wallingford, CT, 2004;
- 15bsingle point energy calculations with the M05-2X and M06-2X functionals were run with NWChem. 5.0: E. J. Bylaska et al., NWChem, version 5.0, Pacific Northwest National Laboratory, Richland, WA, 2006;
- 15cgeometries and unscaled zero point energy corrections of all the structures were obtained with each DFT method and the 6-31+G(d,p) basis set. For M05-2X, M06-2X and SCS-MP2, the electronic energies were computed by single point energy calculations on the B3LYP/6-31+G(d,p) geometries and added zero point energy corrections also computed at the latter level of theory (see Supporting Information for full computational details).
- 16
- 16aM. R. Nyden, G. A. Petersson, J. Chem. Phys. 1981, 75, 1843–1862;
- 16bG. A. Petersson, M. A. Al-Laham, J. Chem. Phys. 1991, 94, 6081–6090;
- 16cG. A. Petersson, T. Tensfeldt, J. A. Montgomery, Jr., J. Chem. Phys. 1991, 94, 6091–6101;
- 16dJ. A. Montgomery, Jr., J. W. Ochterski, G. A. Petersson, J. Chem. Phys. 1994, 101, 5900–5909.
- 17L. A. Curtiss, K. Raghavachari, P. C. Redfern, V. Rassolov, J. A. Pople, J. Chem. Phys. 1998, 109, 7764–7776.
- 18
- 18aB3LYP: ref. [2];
- 18bB1B95: A. D. Becke, J. Chem. Phys. 1996, 104, 1040–1046;
- 18cmPW1PW91: C. Adamo, V. Barone, J. Chem. Phys. 1998, 108, 664–675;
- 18dMPW1K: B. J. Lynch, P. L. Fast, M. Harris, D. G. Truhlar, J. Phys. Chem. A 2000, 104, 4811–4815;
- 18eMPW1B95 and MPWB1K: Y. Zhao, D. G. Truhlar, J. Phys. Chem. A 2004, 108, 6908–6918;
- 18fM05-2X: Y. Zhao, N. E. Schultz, D. G. Truhlar, J. Chem. Theory Comput. 2006, 2, 364–382;
- 18gM06-2X: Y. Zhao, D. G. Truhlar, Theor. Chem. Acc. 2008, 120, 215–241.
- 19
- 19aS. Grimme, J. Chem. Phys. 2003, 118, 9095–9102;
- 19bstandard MP2 results are given in the Supporting Information.
- 20Experimental values are computed from NIST (http://webbook.nist.gov/chemistry/) ΔHf at 298 K and corrected to 0 K using the B3LYP/6-31+G(d,p) thermal energy corrections.
- 21
- 21aW. J. Hehre, R. Ditchfield, L. Radom, J. A. Pople, J. Am. Chem. Soc. 1970, 92, 4796–4801;
- 21bL. Radom, W. J. Hehre, J. A. Pople, J. Am. Chem. Soc. 1971, 93, 289–300.