Agostic NSiH⋅⋅⋅Mo Complexes: From Curiosity to Catalysis†
Andrey Y. Khalimon Dipl.-Chem.
Chemistry Department, Brock University, 500 Glenridge Ave., St. Catharines, ON L2S 3A1 (Canada), Fax: (+1) 905-682-9020
Search for more papers by this authorRazvan Simionescu Dipl.-Ing.
Chemistry Department, Brock University, 500 Glenridge Ave., St. Catharines, ON L2S 3A1 (Canada), Fax: (+1) 905-682-9020
Search for more papers by this authorLyudmila G. Kuzmina Prof.
Institute of General and Inorganic Chemistry RAS, Moscow (Russia)
Search for more papers by this authorJudith A. K. Howard Prof.
Chemistry Department, University of Durham (UK)
Search for more papers by this authorGeorgii I. Nikonov Dr.
Chemistry Department, Brock University, 500 Glenridge Ave., St. Catharines, ON L2S 3A1 (Canada), Fax: (+1) 905-682-9020
Search for more papers by this authorAndrey Y. Khalimon Dipl.-Chem.
Chemistry Department, Brock University, 500 Glenridge Ave., St. Catharines, ON L2S 3A1 (Canada), Fax: (+1) 905-682-9020
Search for more papers by this authorRazvan Simionescu Dipl.-Ing.
Chemistry Department, Brock University, 500 Glenridge Ave., St. Catharines, ON L2S 3A1 (Canada), Fax: (+1) 905-682-9020
Search for more papers by this authorLyudmila G. Kuzmina Prof.
Institute of General and Inorganic Chemistry RAS, Moscow (Russia)
Search for more papers by this authorJudith A. K. Howard Prof.
Chemistry Department, University of Durham (UK)
Search for more papers by this authorGeorgii I. Nikonov Dr.
Chemistry Department, Brock University, 500 Glenridge Ave., St. Catharines, ON L2S 3A1 (Canada), Fax: (+1) 905-682-9020
Search for more papers by this authorThis work was supported by the Petroleum Research Fund, administered by the American Chemical Society. L.G.K. thanks the Russian Foundation of Basic Research.
Graphical Abstract
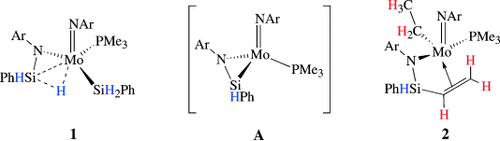
An uncommon catalyst: The β-agostic NSiH⋅⋅⋅M complex 1 (Ar=2,6-diisopropylphenyl) catalyzes a variety of hydrosilylation reactions. Stoichiometric reactions of 1 with unsaturated compounds proceed via the silanimine intermediate A and, in the case of olefins or nitriles, give products of SiC coupling, such as 2.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z802147_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For agostic d0 silylamido complexes, see:
- 1aW. A. Herrmann, N. W. Huberand, J. Behm, Chem. Ber. 1992, 125, 1405;
- 1bL. J. Procopio, P. J. Carroll, D. H. Berry, J. Am. Chem. Soc. 1994, 116, 177;
- 1cW. A. Herrmann, J. Eppinger, M. Spiegler, O. Runte, R. Anwander, Organometallics 1997, 16, 1813;
- 1dI. Nagl, W. Scherer, M. Tafipolsky, R. Anwander, Eur. J. Inorg. Chem. 1999, 1405;
10.1002/(SICI)1099-0682(199909)1999:9<1405::AID-EJIC1405>3.0.CO;2-# CAS Web of Science® Google Scholar
- 1eJ. Eppinger, M. Spiegler, W. Hieringer, W. A. Herrmann, R. Anwander, J. Am. Chem. Soc. 2000, 122, 3080.
- 2For agostic d2 silylamido complexes, see:
- 2aG. I. Nikonov, P. Mountford, S. K. Ignatov, J. C. Green, P. A. Cooke, M. A. Leech, L. G. Kuzmina, A. G. Razuvaev, N. H. Rees, A. J. Blake, J. A. K. Howard, D. A. Lemenovskii, J. Chem. Soc. Dalton Trans. 2001, 2903;
- 2bS. K. Ignatov, N. H. Rees, S. R. Dubberley, A. G. Razuvaev, P. Mountford, G. I. Nikonov, Chem. Commun. 2004, 952.
- 3G. I. Nikonov, Adv. Organomet. Chem. 2005, 53, 217.
- 4S. K. Ignatov, N. H. Rees, A. A. Merkoulov, S. R. Dubberley, A. G. Razuvaev, P. Mountford, G. I. Nikonov, Chem. Eur. J. 2008, 14, 296.
- 5
- 5aL. J. Procopio, P. J. Carroll, D. H. Berry, J. Am. Chem. Soc. 1991, 113, 1870;
- 5bL. J. Procopio, P. J. Carroll, D. H. Berry, Polyhedron 1995, 14, 45;
- 5cU. Burckhardt, G. L. Casty, T. D. Tilley, T. W. Woo, U. Rothlisberger, Organometallics 2000, 19, 3830.
- 6L. J. Procopio, P. J. Carroll, D. H. Berry, Organometallics 1993, 12, 3087.
- 7The energy barrier to activation of the SiH bond depends on the extent of back donation.[3] Assuming a triple-bond character for the imido group, 2 a,b are 18-electron complexes, whereas 3 is a 16-electron complex.
- 8See the following reviews and references therein:
- 8aJ.-F. Carpentier, V. Bette, Curr. Org. Chem. 2002, 6, 913;
- 8bO. Riant, N. Mostefai, J. Courmarcel, Synthesis 2004, 2943;
- 8cB. Marciniec, J. Gulinski, J. Organomet. Chem. 1993, 446, 15;
- 8dS. E. Gibson, M. Rudd, Adv. Synth. Catal. 2007, 349, 781;
- 8eB. Marciniec, Appl. Organomet. Chem. 2000, 14, 527;
- 8fI. Ojima in The Chemistry of Organic Silicon Compounds (Eds.: ), Wiley, New York, 1989, chap. 25;
10.1002/0470025107.ch25 Google Scholar
- 8gB. Marciniec, Comprehensive Handbook on Hydrosilylation, Pergamon, Oxford, 1992.
- 9A. D. Sadow, T. D. Tilley, Organometallics 2001, 20, 4457.
- 10For reduction of carbonyl compounds to alkanes, see:
- 10aC. T. West, S. J. Donnelly, D. A. Kooistra, M. P. Doyle, J. Org. Chem. 1973, 38, 2675;
- 10bL. R. C. Barclay, H. R. Sonawane, M. C. MacDonald, Can. J. Chem. 1972, 50, 281;
- 10cJ. L. Fry, M. Orfanopoulos, M. G. Adlington, W. R. Dittman, Jr., S. B. Silverman, J. Org. Chem. 1978, 43, 374.
- 11
- 11aM. P. Doyle, C. C. McOsker, J. Org. Chem. 1978, 43, 693;
- 11bF. A. Carey, H. S. Tremper, J. Org. Chem. 1969, 34, 4.
- 12For example:
- 12aC. J. Yue, Y. Liu, R. He, J. Mol. Catal. A 2006, 259, 17;
- 12bR. L. Stapleton, J. Chai, N. J. Taylor, S. Collins, Organometallics 2006, 25, 2514.
- 13J. Ejfler, M. Kobulka, M. Hojniak, P. Sobota, J. Mol. Catal. A 2004, 224, 93.
- 14For example:
- 14aY. Gunji, Y. Yamashita, T. Ikeno, T. Yamada, Chem. Lett. 2006, 35, 714;
- 14bD. Field, B. A. Messerle, M. Rher, L. P. Soler, T. W. Hambley, Organometallics 2003, 22, 2387;
- 14cU. Schubert, C. Lorenz, Chem. Ber. 1995, 128, 1267;
- 14dT. C. Bedard, J. Y. Corey, J. Organomet. Chem. 1992, 428, 315;
- 14eD. H. R. Barton, M. J. Kelly, Tetrahedron Lett. 1992, 33, 5041.
- 15
- 15aR. Calas, E. Frainnet, A. Bazouin, C. R. Hebd. Seances Acad. Sci. 1961, 252, 420;
- 15bA. J. Chalk, J. Organomet. Chem. 1970, 21, 207;
- 15cR. J. P. Corriu, J. J. E. Moreau, M. Pataud-Sat, J. Organomet. Chem. 1982, 228, 301;
- 15dT. Murai, T. Sakane, S. Kato, J. Org. Chem. 1990, 55, 449;
- 15eA. M. Caporusso, N. Panziera, P. Pertici, E. Pitzalis, P. Salvadori, G. Vitulli, G. Martra, J. Mol. Catal. A 1999, 150, 275;
- 15fJ. Kim, Y. Kang, J. Lee, Y. K. Kong, M. S. Gong, S. O. Kang, J. Ko, Organometallics 2001, 20, 937;
- 15gH. Hashimoto, I. Aratani, C. Kabuto, M. Kira, Organometallics 2003, 22, 2199;
- 15hM. Ochiai, H. Hashimoto, H. Tobita, Angew. Chem. 2007, 119, 8340;
10.1002/ange.200703154 Google ScholarAngew. Chem. Int. Ed. 2007, 46, 8192;
- 15iN. W. Mitzel, J. Riede, A. Schier, M. Paul, H. Schmidbaur, Chem. Ber. 1993, 126, 2027.
- 16B. H. Kim, H.-G. Woo, Adv. Organomet. Chem. 2004, 52, 143.
- 17For an example of η2 coordination, see: I. J. Blackmore, C. J. S. Emiao, M. S. A. Buschhaus, B. O. Patric, P. Legzdins, Organometallics 2007, 26, 4881.
- 18For examples of η1 coordination, see:
- 18aJ. Yang, M. Brookhart, J. Am. Chem. Soc. 2007, 129, 12656;
- 18bC. S. Weinert, Organometallics 2005, 24, 5759;
- 18cB. Müller, H. Vahrenkamp, Eur. J. Inorg. Chem. 1999, 117;
- 18dJ. S. Song, D. J. Szalda, R. M. Bullock, Inorg. Chim. Acta 1997, 259, 161;
- 18eM. Bochmann, K. J. Webb, M. B. Hursthouse, M. Mazid, J. Chem. Soc. Chem. Commun. 1991, 1735.
- 19A. B. Jackson, C. K. Schauer, P. S. White, J. L. Templeton, J. Am. Chem. Soc. 2007, 129, 10628.
- 20Rearrangement of the methyl derivative 12 is complicated, owing to the additional activation of the CH bond of the CH3CN ligand. For CH bond activation in nitriles, see:
- 20aH. Takaya, S.-I. Murahashi, Synlett 2001, 991;
- 20bH. J. Heeres, A. Meetsma, J. H. Teuben, Angew. Chem. 1990, 102, 449; Angew. Chem. Int. Ed. Engl. 1990, 29, 420;
- 20cT. Naota, A. Tannna, S.-I. Murahashi, Chem. Commun. 2001, 63;
- 20dS.-I. Murahashi, H. Takaya, T. Naota, Pure Appl. Chem. 2002, 74, 19;
- 20eT. A. Atesin, T. Li, S. Lachaize, W. W. Brennessel, J. J. Garcia, W. D. Jones, J. Am. Chem. Soc. 2007, 129, 7562;
- 20fE. Fujita, C. Creutz, Inorg. Chem. 1994, 33, 1729.