Carboxylate-Bridged Dinuclear Active Sites in Oxygenases: Diiron, Dimanganese, or is Heterodinuclear Better?
Graphical Abstract
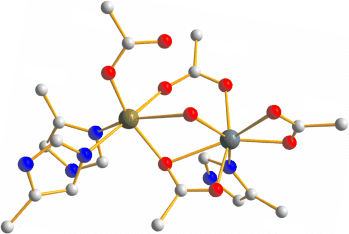
To mix or not to mix: The N-oxygenase AurF of S. thioluteus has an unusual carboxylate-bridged dinuclear active site (see picture; gray C, blue N, red O, green Mn, turquoise Mn/Fe). The similarity with a recently discovered Mn/Fe-oxygenase subunit of the ribonucleotide reductase of C. trachomatis suggests that it might contain both Mn and Fe. The N-oxygenase of S. thioluteus very likely represents the first member of a new family of Mn/Fe-oxygenases.