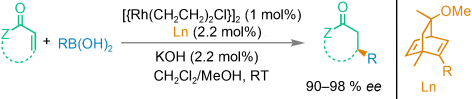C1-Symmetric Monosubstituted Chiral Diene Ligands in Asymmetric Rhodium-Catalyzed 1,4-Addition Reactions†
Thomas Gendrineau
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorOlivier Chuzel Dr.
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorHendrik Eijsberg
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorJean-Pierre Genet Prof.
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorSylvain Darses Dr.
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorThomas Gendrineau
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorOlivier Chuzel Dr.
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorHendrik Eijsberg
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorJean-Pierre Genet Prof.
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorSylvain Darses Dr.
Laboratoire de Synthèse Sélective Organique (UMR 7573), Ecole Nationale Supérieure de Chimie de Paris, 11 rue P&M Curie, 75231 Paris cedex 05 (France), Fax: (+33) 1-4407-1062
Search for more papers by this authorThis work was support by the Centre National de la Recherche Scientifique (CNRS). T. Gendrineau thanks the Ministère de l'Education et de la Recherche for a grant.
Graphical Abstract
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z803230_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1a Asymmetric Catalysis in Organic Synthesis (Ed.: ), Wiley, New York, 1994;
- 1b Catalytic Asymmetric Synthesis (Ed.: ), Wiley, New York, 2000.
10.1002/0471721506 Google Scholar
- 2Review: C. Defieber, H. Grützmacher, E. M. Carreira, Angew. Chem. 2008, 120, 4558–4579;
10.1002/ange.200703612 Google ScholarAngew. Chem. Int. Ed. 2008, 47, 4482–4502.
- 3C. Fischer, C. Defieber, T. Suzuki, E. M. Carreira, J. Am. Chem. Soc. 2004, 126, 1628–1629.
- 4T. Hayashi, K. Ueyama, N. Tokunaga, K. Yoshida, J. Am. Chem. Soc. 2003, 125, 11508–11509.
- 5Rhodium-catalyzed 1,4-additions by using chiral phosphorous ligands:
- 5aM. Sakai, H. Hayashi, N. Miyaura, Organometallics 1997, 16, 4229–4231;
- 5bY. Takaya, M. Ogasawara, T. Hayashi, M. Sakai, N. Miyaura, J. Am. Chem. Soc. 1998, 120, 5579–5580. Reviews on rhodium-catalyzed asymmetric 1,4-addition:
- 5cT. Hayashi, Synlett 2001, 879–887;
- 5dT. Hayashi, K. Yamasaki, Chem. Rev. 2003, 103, 2829–2844;
- 5eT. Hayashi, Bull. Chem. Soc. Jpn. 2004, 77, 13–21;
- 5fT. Hayashi, Pure Appl. Chem. 2004, 76, 465–475.
- 6The use of C2-symmetric chiral dienes in asymmetric rhodium-catalyzed addition to imines:
- 6aN. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2004, 126, 13584–13585;
- 6bY. Otomaru, N. Tokunaga, R. Shintani, T. Hayashi, Org. Lett. 2005, 7, 307–310;
- 6cY. Otomaru, A. Kina, R. Shintani, T. Hayashi, Tetrahedron: Asymmetry 2005, 16, 1673–1679;
- 6dT. Nishimura, Y. Yasuhara, T. Hayashi, Org. Lett. 2006, 8, 979–981.
- 7Ligands derived from bicyclo[2.2.1]heptane structures:
- 7aG. Berthon-Gelloz, T. Hayashi, J. Org. Chem. 2006, 71, 8957–8960;
- 7bT. Noel, K. Vandyck, J. Van der Eycken, Tetrahedron 2007, 63, 12961–12967. Bicyclo[2.2.2]octadienes:
- 7cR. Shintani, A. Tsurusaki, K. Okamoto, T. Hayashi, Angew. Chem. 2005, 117, 3977–3980;
10.1002/ange.200500843 Google ScholarAngew. Chem. Int. Ed. 2005, 44, 3909–3912;
- 7dT. Hayashi, N. Tokunaga, K. Okamoto, R. Shintani, Chem. Lett. 2005, 34, 1480–1481;
- 7eY. Otomaru, K. Okamoto, R. Shintani, T. Hayashi, J. Org. Chem. 2005, 70, 2503–2508;
- 7fR. Shintani, K. Okamoto, T. Hayashi, Org. Lett. 2005, 7, 4757–4759;
- 7gF. Chen, A. Kina, T. Hayashi, Org. Lett. 2006, 8, 341–344;
- 7hY. Nakao, J. Chen, H. Imanaka, T. Hiyama, Y. Ichikawa, W. L. Duan, R. Shintani, T. Hayashi, J. Am. Chem. Soc. 2007, 129, 9137–9143;
- 7iW. L. Duan, Y. Imazaki, R. Shintani, T. Hayashi, Tetrahedron 2007, 63, 8529–8536;
- 7jR. Shintani, Y. Ichikawa, T. Hayashi, J. Chen, Y. Nakao, T. Hiyama, Org. Lett. 2007, 9, 4643–4645;
- 7kS. Sörgel, N. Tokunaga, K. Sasaki, K. Okamoto, T. Hayashi, Org. Lett. 2008, 10, 589–592. Bicyclo[3.3.1]nona-2,6-diene:
- 7lY. Otomaru, A. Kina, R. Shintani, T. Hayashi, Tetrahedron: Asymmetry 2005, 16, 1673–1679;
- 7mY. Nakao, M. Takeda, J. Chen, T. Hiyama, Y. Ichikawa, R. Shintani, T. Hayashi, Chem. Lett. 2008, 37, 290–291. 1,5-cyclooctadiene:
- 7nA. Kina, K. Ueyama, T. Hayashi, Org. Lett. 2005, 7, 5889–5892.
- 8C2-type symmetric bicyclo[2.2.2]octadienes derived from (−)-carvone:
- 8aC. Defieber, J. Paquin, S. Serna, E. M. Carreira, Org. Lett. 2004, 6, 3873–3876;
- 8bJ. Paquin, C. Defieber, C. R. J. Stephenson, E. M. Carreira, J. Am. Chem. Soc. 2005, 127, 10850–10851;
- 8cJ. Paquin, C. R. J. Stephenson, C. Defieber, E. M. Carreira, Org. Lett. 2005, 7, 3821–3824.
- 9Bicyclo[3.3.0]octa-2,5-dienes:
- 9aS. Helbig, S. Sauer, N. Cramer, S. Laschat, A. Baro, W. Frey, Adv. Synth. Catal. 2007, 349, 2331–2337;
- 9bZ. Wang, C. G. Feng, M. Xu, G. Q. Lin, J. Am. Chem. Soc. 2007, 129, 5336–5337.
- 10An example of C1-symmetric chiral diene in rhodium-catalyzed 1,4-addition (moderate ee values observed): F. Läng, F. Breher, D. Stein, H. Grützmacher, Organometallics 2005, 24, 2997–3007. Carreira and co-workers also reported the low efficiency of such monosubstituted chiral dienes: see reference [8a].
- 11
- 11aM. Pucheault, S. Darses, J. P. Genet, Eur. J. Org. Chem. 2002, 3552–3557;
- 11bL. Navarre, S. Darses, J. P. Genet, Angew. Chem. 2004, 116, 737–741;
10.1002/ange.200352518 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 719–723;
- 11cL. Navarre, S. Darses, J. P. Genet, Chem. Commun. 2004, 1108–1109;
- 11dM. Pucheault, S. Darses, J. P. Genet, J. Am. Chem. Soc. 2004, 126, 15356–15357;
- 11eM. Pucheault, S. Darses, J. P. Genet, Chem. Commun. 2005, 4714–4716;
- 11fG. Mora, S. Darses, J. P. Genet, Adv. Synth. Catal. 2007, 349, 1180–1184;
- 11gR. Martinez, F. Voica, J. P. Genet, S. Darses, Org. Lett. 2007, 9, 3213–3216.
- 12A. Srikrishna, G. V. R. Sharma, S. Danieldoss, P. Hemamalini, J. Chem. Soc. Perkin Trans. 1 1996, 1305–1311.
- 13D. L. Comins, A. Dehghani, Tetrahedron Lett. 1992, 33, 6299–6302.
- 14
- 14aG. A. Molander, D. E. Petrillo, N. R. Landzberg, J. C. Rohanna, B. Biolatto, Synlett 2005, 1763–1766;
- 14bS. Darses, J. P. Genet, Chem. Rev. 2008, 108, 288–325;
- 14cH. A. Stefani, R. Cella, A. S. Vieira, Tetrahedron 2007, 63, 3623–3658;
- 14dG. A. Molander, N. M. Ellis, Acc. Chem. Res. 2007, 40, 275–286;
- 14eG. A. Molander, R. Figueroa, Aldrichimica Acta 2005, 38, 49–56;
- 14fS. Darses, J. P. Genet, Eur. J. Org. Chem. 2003, 4313–4327.
- 15Review: N. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457–2483.
- 16(SRR)-Ln corresponds to (1S,4R,5R)-Ln for L1–L11 and L13–L14 and to (1S,4R,8R)-Ln for L12.
- 17Improved reaction efficiency was also observed by using an alcoholic solvent instead of water in a related reaction: S. Brock, D. R. J. Hose, J. D. Moseley, A. J. Parker, I. Patel, A. J. Williams, Org. Process Res. Dev. 2008, 12, 496–502.
- 18T. Hayashi, M. Takahashi, Y. Takaya, M. Ogasawara, J. Am. Chem. Soc. 2002, 124, 5052–5058.