Primary-Amine-Catalyzed Enantioselective Intramolecular Aldolizations†
Jian Zhou Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2982
Search for more papers by this authorVijay Wakchaure Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2982
Search for more papers by this authorPhilip Kraft Dr.
Givaudan Schweiz AG, 8600 Dübendorf (Switzerland)
Search for more papers by this authorBenjamin List Prof. Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2982
Search for more papers by this authorJian Zhou Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2982
Search for more papers by this authorVijay Wakchaure Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2982
Search for more papers by this authorPhilip Kraft Dr.
Givaudan Schweiz AG, 8600 Dübendorf (Switzerland)
Search for more papers by this authorBenjamin List Prof. Dr.
Max-Planck-Institut für Kohlenforschung, Kaiser Wilhelm-Platz 1, 45470 Mülheim an der Ruhr (Germany), Fax: (+49) 208-306-2982
Search for more papers by this authorThe authors acknowledge generous funding from the Max Planck Society, the DFG (SPP 1179, Organocatalysis), the Fonds der Chemischen Industrie, and Wacker Chemie AG.
Graphical Abstract
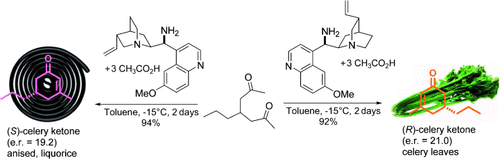
Aldol cyclodehydration of 4-substituted-2,6-heptanediones leads to enantiomerically enriched 5-substituted-3-methyl-2-cyclohexene-1-ones, which serve as perfume ingredients and valuable synthetic building blocks. Primary amines derived from cinchona alkaloids in combination with acetic acid are efficient catalysts for this transformation, which deliver both enantiomers of the celery ketone.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z802497_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1
- 1aC. Agami, H. Sevestre, J. Chem. Soc. Chem. Commun. 1984, 1385–1386;
- 1bC. Agami, N. Platzer, H. Sevestre, Bull. Soc. Chim. Fr. 1987, 2, 358–360.
- 2
- 2aZ. G. Hajos, D. R. Parrish, Germany Patent DE 21022623, 1971;
- 2bU. Eder, G. R. Sauer, R. Wiechert, Germany Patent DE 2014757, 1971;
- 2cU. Eder, G. Sauer, R. Wiechert, Angew. Chem. 1971, 83, 492;
10.1002/ange.19710831307 Google ScholarAngew. Chem. Int. Ed. Engl. 1971, 10, 496;
- 2dG. Hajos, D. R. Parrish, J. Org. Chem. 1974, 39, 1615—1621. For a general review, see:
- 2eS. Mukherjee, J. W. Yang, S. Hoffmann, B. List, Chem. Rev. 2007, 107, 5471–5569.
- 3B. List, R. A. Lerner, C. F. Barbas III, Org. Lett. 1999, 1, 59–61.
- 4See, for example:
- 4aC. S. Letizia, J. Cocchiara, G. A. Wellington, C. Funk, A. M. Api, Food Chem. Toxicol. 2000, 38, S 153–S155;
- 4bM. A. González, S. Ghosh, F. Rivas, D. Fischer, E. A. Theodorakis, Tetrahedron Lett. 2004, 45, 5039–5041;
- 4cB. H. Bae, K. S. Im, W. C. Choi, J. Hong, C.-O. Lee, J. S. Choi, B. W. Son, J.-I. Song, J. H. Jung, J. Nat. Prod. 2000, 63, 1511–1514;
- 4dH. Yamamoto, H. L. Sham, J. Am. Chem. Soc. 1979, 101, 1609–1611.
- 5For examples of asymmetric enamine catalysis using primary amines, see:
- 5aS. Danishefsky, P. Cain, J. Am. Chem. Soc. 1976, 98, 4975–4983;
- 5bS. G. Davies, R. L. Sheppard, A. D. Smith, J. E. Thomson, Chem. Commun. 2005, 3802–3804;
- 5cW. Zou, I. Ibrahem, P. Dziedzic, H. Sundén, A. Córdova, Chem. Commun. 2005, 4946–4948;
- 5dM. Amedjkouh, Tetrahedron: Asymmetry 2005, 16, 1411–1414;
- 5eS. B. Tsogoeva, S. Wei, Chem. Commun. 2006, 1451–1453;
- 5fH. Huang, E. N. Jacobsen, J. Am. Chem. Soc. 2006, 128, 7170–7171;
- 5gT.-Y. Liu, H.-L. Cui, Y. Zhang, K. Jiang, W. Du, Z.-Q. He, Y.-C. Chen, Org. Lett. 2007, 9, 3671–3674;
- 5hS. H. McCooey, S. J. Conon, Org. Lett. 2007, 9, 599–602;
- 5iS. S. V. Ramasastry, H. Zhang, F. Tanaka, C. F. Barbas III, J. Am. Chem. Soc. 2007, 129, 288–289;
- 5jS. Luo, H. Xu, J. Li, L. Zhang, J.-P. Cheng, J. Am. Chem. Soc. 2007, 129, 3074–3075;
- 5kB.-L. Zheng, Q.-Z. Liu, C.-S. Guo, X.-L. Wang, L. He, Org. Biomol. Chem. 2007, 5, 2913–2915. For a review on primary amino acids as catalysts, see:
- 5lL.-W. Xu, Y. Lu, Org. Biomol. Chem. 2008, 6, 2047–2053.
- 6For examples of asymmetric iminium ion catalysis using primary amines, see:
- 6aK. Ishihara, K. Nakano, J. Am. Chem. Soc. 2005, 127, 10504–10505;
- 6bN. J. A. Martin, B. List, J. Am. Chem. Soc. 2006, 128, 13368–13369;
- 6cH. Kim, C. Yen, P. Preston, J. Chin, Org. Lett. 2006, 8, 5239–5242;
- 6dJ.-W. Xie, W. Chen, R. Li, M. Zeng, W. Du, L. Yue, Y.-C. Chen, Y. Wu, J. Zhu, J.-G. Deng, Angew. Chem. 2007, 119, 393–396; Angew. Chem. Int. Ed. 2007, 46, 389–392;
- 6eG. Bartoli, M. Bosco, A. Carlone, F. Pesciaioli, L. Sambri, P. Melchiorre, Org. Lett. 2007, 9, 1403–1405;
- 6fR. P. Singh, K. Bartelson, Y. Wang, H. Su, X. Lu, L. Deng, J. Am. Chem. Soc. 2008, 130, 2422–2423;
- 6gX. Wang, C. M. Reisinger, B. List, J. Am. Chem. Soc. 2008, 130, 6070–6071.
- 7For enamine catalysis of intermolecular aldolizations of ketones, see:
- 7aO. Tokuda, T. Kano, W.-G. Gao, T. Ikemoto, K. Maruoka, Org. Lett. 2005, 7, 5103–5105;
- 7bS. Samanta, C. G. Zhao, J. Am. Chem. Soc. 2006, 128, 7442–7443;
- 7cZ. Tang, L.-F. Cun, X. Cui, A.-Q. Mi, Y.-Z. Jiang, L.-Z. Gong, Org. Lett. 2006, 8, 1263–1266;
- 7dJ. Liu, Z. Yang, Z. Wang, F. Wang, X. Chen, X. Liu, X. Feng, Z. Su, C. Hu, J. Am. Chem. Soc. 2008, 130, 5654–5655.
- 8S. Bahmanyar, K. N. Houk, J. Am. Chem. Soc. 2001, 123, 11273–11283.
- 9J. Zhou, B. List, J. Am. Chem. Soc. 2007, 129, 7498–7499.
- 10CH3CO2H (pKa=4.76, e.r.=28.1); PhCO2H (pKa=4.31, e.r.=17.8); Cl3CCO2H (pKa=0.65, e.r.=16.5); CF3CO2H (pKa=−0.25, e.r.=9.8); pTsOH (pKa=−2.80, e.r.=4.9). For a discussion of acid co-catalyst effects in enamine catalytic aldolizations, see: A. Erkkilä, P. M. Pihko, Eur. J. Org. Chem. 2007, 4205–4216.
- 11
- 11aX. Z. Zhao, L. Peng, M. Tang, Y. Q. Tu, S. H. Gao, Tetrahedron Lett. 2005, 46, 6941–6944;
- 11bY. X. Jia, X. Li, B. Wu, X. Z. Zhao, Y. Q. Tu, Tetrahedron 2002, 58, 1697–1708;
- 11cY. X. Jia, B. Wu, X. Li, S. K. Ren, Y. Q. Tu, A. S. C. Chan, W. Kitching, Org. Lett. 2001, 3, 847–849.
- 12N. L. Allinger, C. K. Riew, J. Org. Chem. 1975, 40, 1316–1321.
- 13For the one-step synthesis of diketone 2 a, see the Supporting Information.
- 14
- 14aL. Friedman, J. G. Miller, Science 1971, 172, 1044–1046;
- 14bC. S. Sell, Chem. Biodiversity 2004, 1, 1899–1920;
- 14cP. Kraft, G. Fráter, Chirality 2001, 13, 388–394.
- 15For a review, see: S. Saito, H. Yamamoto, Acc. Chem. Res. 2004, 37, 570–579.