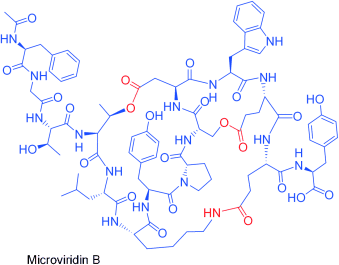
Ribosomal Synthesis of Tricyclic Depsipeptides in Bloom-Forming Cyanobacteria†
We thank M.-G. Schwinger for E. coli mass culture, Dr. R. Winkler and M. Pötsch for MALDI-TOF analyses, A. Perner for HRMS and HPLC/MS measurements, R. Günther, K. Hinrichs, and J. Müller for their assistance with the molecular work, and U. Newen, Leibniz-Institute for Freshwater Ecology and Inland Fisheries, Berlin. Financial support by the Leibniz Gemeinschaft, the DFG (ILRS, JSMC, SPP1152) and the German–Israeli Foundation (GIF) is gratefully acknowledged.
Graphical Abstract
Decoding biosynthesis: Cage-like depsipeptides have been investigated by a combination of genetic and chemical analyses. Heterologous expression and MALDI-PSD studies reveal that the cyanobacterial protease inhibitors microviridin B and J are synthesized from ribosomally produced prepeptides that are transformed into tricyclic depsipeptides by ATP grasp ligases and processed by a transporter peptidase.