Synthesis of Biaryl Compounds through Three-Component Assembly: Ambidentate Effect of the tert-Butyldimethylsilyl Group for Regioselective Diels–Alder and Hiyama Coupling Reactions†
Shuji Akai Prof. Dr.
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorTakashi Ikawa Dr.
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorSho-ichi Takayanagi
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorYuki Morikawa
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorShinya Mohri
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorMasaya Tsubakiyama
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorMasahiro Egi Dr.
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorYasufumi Wada
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6, Yamadaoka, Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorYasuyuki Kita Prof. Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6, Yamadaoka, Suita, Osaka 565-0871 (Japan)
Current address: School of Pharmaceutical Sciences, Ritsumeikan University, 1-1-1 Noji Higashi, Kusatsu, Shiga 525-8577 (Japan)
Search for more papers by this authorShuji Akai Prof. Dr.
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorTakashi Ikawa Dr.
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorSho-ichi Takayanagi
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorYuki Morikawa
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorShinya Mohri
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorMasaya Tsubakiyama
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorMasahiro Egi Dr.
School of Pharmaceutical Sciences, University of Shizuoka, 52-1, Yada, Suruga-ku, Shizuoka, Shizuoka 422-8526 (Japan), Fax: (+81) 54-264-5672
Search for more papers by this authorYasufumi Wada
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6, Yamadaoka, Suita, Osaka 565-0871 (Japan)
Search for more papers by this authorYasuyuki Kita Prof. Dr.
Graduate School of Pharmaceutical Sciences, Osaka University, 1-6, Yamadaoka, Suita, Osaka 565-0871 (Japan)
Current address: School of Pharmaceutical Sciences, Ritsumeikan University, 1-1-1 Noji Higashi, Kusatsu, Shiga 525-8577 (Japan)
Search for more papers by this authorThis work was supported by Grants-in-Aid for Scientific Research and the global COE program from the Ministry of Education, Culture, Sports, Science, and Technology (Japan). We thank the Uehara Memorial Foundation for financial support.
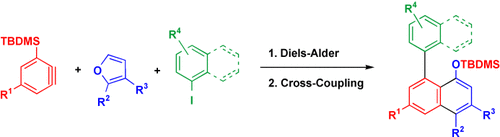
Graphical Abstract
Two for the price of one: A method has been developed for the regiocontrolled synthesis of multisubstituted biaryl derivatives. This protocol involves the use of the tert-butyldimethylsilyl (TBDMS) group to direct the regioselective Diels–Alder reaction of a 3-TBDMS-benzyne with a furan derivative and a subsequent Hiyama cross-coupling reaction of the TBDMS group with aryl iodides (see scheme).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2008/z803011_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1aK. B. G. Torssell, Natural Product Chemistry, Wiley, Chichester, 1983;
- 1bR. H. Thomson, The Chemistry of Natural Products, Blackie and Son, Glasgow, 1985;
- 1cO. Baudoin, F. Guéritte, Stud. Nat. Prod. Chem. 2003, 29, 355–417;
- 1dA. V. R. Rao, M. K. Gurjar, K. L. Reddy, A. S. Rao, Chem. Rev. 1995, 95, 2135–2167;
- 1eK. C. Nicolaou, C. N. C. Boddy, S. Bräse, N. Winssinger, Angew. Chem. 1999, 111, 2230–2287;
10.1002/(SICI)1521-3757(19990802)111:15<2230::AID-ANGE2230>3.0.CO;2-V Google ScholarAngew. Chem. Int. Ed. 1999, 38, 2096–2152;10.1002/(SICI)1521-3773(19990802)38:15<2096::AID-ANIE2096>3.0.CO;2-F CAS PubMed Web of Science® Google Scholar
- 1fJ. Roncali, Chem. Rev. 1992, 92, 711–738;
- 1gX. Mei, C. Wolf, J. Am. Chem. Soc. 2006, 128, 13326–13327;
- 1hJ. M. Brunel, Chem. Rev. 2005, 105, 857–897;
- 1iM. Berthod, G. Mignani, G. Woodward, M. Lemaire, Chem. Rev. 2005, 105, 1801–1836.
- 2
- 2a Handbook of Organopalladium Chemistry for Organic Synthesis (Eds.: ), Wiley Interscience, New York, 2002;
- 2b Metal-Catalyzed Cross-Coupling Reactions, Vols. 1 and 2 (Eds.: ), Wiley-VCH, Weinheim, 2004.
- 3For recent examples, see:
- 3aK. Tonogaki, K. Itami, J. Yoshida, J. Am. Chem. Soc. 2006, 128, 1464–1465;
- 3bS.-X. Wang, M.-X. Wang, D.-X. Wang, J. Zhu, Angew. Chem. 2008, 120, 394–397; Angew. Chem. Int. Ed. 2008, 47, 388–391.
- 4For recent reviews on benzynes, see:
- 4aH. Pellissier, M. Santelli, Tetrahedron 2003, 59, 701–730;
- 4bH. H. Wenk, M. Winkler, W. Sander, Angew. Chem. 2003, 115, 518–546;
10.1002/ange.200390119 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 502–528;
- 4cA. M. Dyke, A. J. Hester, G. C. Lloyd-Jones, Synthesis 2006, 4093–4112.
- 5Regioselective Diels–Alder reactions of benzynes controlled by their C3 substituents have been limited to the 3-fluoro-[5a] and 3-alkoxybenzynes,[5b,c] which produced cycloadducts with a good to excellent syn regioselectivity:
- 5aG. W. Gribble, D. J. Keavy, S. E. Branz, W. J. Kelly, M. A. Pals, Tetrahedron Lett. 1988, 29, 6227–6230;
- 5bT. Matsumoto, T. Hosoya, M. Katsuki, K. Suzuki, Tetrahedron Lett. 1991, 32, 6735–6736;
- 5cR. G. F. Giles, A. B. Hughes, M. V. Sargent, J. Chem. Soc. Perkin Trans. 1 1991, 1581–1587.
- 6The Diels–Alder reactions of the 3-alkyl- or 3-aryl benzynes provided mixtures of the syn and anti adducts in various ratios, the regioselectivity of which could not be predicted, see:
- 6aJ. E. Anderson, R. W. Franck, W. L. Mandella, J. Am. Chem. Soc. 1972, 94, 4608–4614;
- 6bM. S. Newman, R. Kannan, J. Org. Chem. 1976, 41, 3356–3359.
- 7The Diels–Alder reaction between 3-fluoro-6-(trimethylsilyl)benzyne and 2-(trimethylsilyl)furan (2 e) has been reported to give exclusively the 1,5-bis(trimethylsilyl)-1,4-epoxy-8-fluoro-1,4-dihydronaphthalene. However, the Diels–Alder reaction of the simple 3-(trimethylsilyl)benzyne was not examined. It was also reported that the trimethylsilyl group was cleaved during the acid-catalyzed opening of the 1,4-epoxide of another Diels–Alder product, see: E. Masson, M. Schlosser, Eur. J. Org. Chem. 2005, 4401–4405.
- 8For the regioselective dipolar [2+3] cycloaddition reactions of 3-silylated benzynes with nitrones, see: T. Matsumoto, T. Sohma, S. Hatazaki, K. Suzuki, Synlett 1993, 843–846.
- 9For some examples of acid-catalyzed opening of 1,4-epoxy-1,4-dihydronaphthalenes, see:
- 9aD. G. Batt, D. G. Jones, S. La Greca, J. Org. Chem. 1991, 56, 6704–6708;
- 9bM. Schlosser, E. Castagnetti, Eur. J. Org. Chem. 2001, 3991–3997;
- 9cD. E. Kaelin, S. M. Sparks, H. R. Plake, S. F. Martin, J. Am. Chem. Soc. 2003, 125, 12994–12995;
- 9dS. Sörgel, C. Azap, H.-U. Reißig, Eur. J. Org. Chem. 2006, 4405–4418.
- 10K. Villeneuve, W. Tam, J. Am. Chem. Soc. 2006, 128, 3514–3515.
- 11For examples, see: G. Bringmann, R. Götz, S. Harmsen, J. Holenz, R. Walter, Liebigs Ann. 1996, 2045–2058.
- 12
- 12aT. Hiyama, J. Organomet. Chem. 2002, 653, 58–61;
- 12bS. E. Denmark, M. H. Ober, Aldrichimica Acta 2003, 36, 75–85.
- 13No reaction took place with the methyl ether of 5 a under the same reaction conditions, which strongly suggests that the formation of the silicate 6 is crucial for this coupling reaction.
- 14
- 14aH. Taguchi, K. Ghoroku, M. Tadaki, A. Tsubouchi, T. Takeda, J. Org. Chem. 2002, 67, 8450–8456;
- 14bY. Nakao, H. Imanaka, A. K. Sahoo, A. Yada, T. Hiyama, J. Am. Chem. Soc. 2005, 127, 6952–6953;
- 14cY. Nakao, J. Chen, M. Tanaka, T. Hiyama, J. Am. Chem. Soc. 2007, 129, 11694–11695; for the activation of vinylsilanes by an intramolecular carboxyl group, see:
- 14dM. Shindo, K. Matsumoto, K. Shishido, Synlett 2005, 176–178.
- 15For examples of intermolecular fluoride-free Hiyama reactions, see: E. Alacid, C. Nájera, Adv. Synth. Catal. 2006, 348, 2085–2091.