Journal list menu
Export Citations
Download PDFs
Cover Picture: Expanding and Shrinking Porous Modulation Based on Pillared-Layer Coordination Polymers Showing Selective Guest Adsorption (Angew. Chem. Int. Ed. 25/2004)
- Page: 3205
- First Published: 16 June 2004
To expand or shrink are properties of the new pillared-layer porous coordination polymers shown in the cover picture. These features show that coordination polymers are much softer than generally believed. The adsorption selectivity exhibited by these new zeolite-like materials arises from a simple change in the organic-pillar module, and may find applications in molecular separation techniques. For more information see the Communication by S. Kitagawa et al. on page 3269 ff.
Block-Selected Molecular Recognition and Formation of Polypseudorotaxanes between Poly(propylene oxide)-Poly(ethylene oxide)-Poly(propylene oxide) Triblock Copolymers and α-Cyclodextrin†
- Page: 3215
- First Published: 16 June 2004
Ordered Porous Nanoarchitectures with Specific Functions†
- Pages: 3216-3217
- First Published: 16 June 2004
Bioactive Natural Products. (Series: Studies in Natural Products Chemistry, Vol. 28, Part 1). Edited by Atta-ur-Rahman.
- Pages: 3218-3219
- First Published: 16 June 2004
Fundamental World of Quantum Chemistry. A Tribute to the Memory of Per-Olov Löwdin. Vols. I and II. Edited by Erkki J. Brändas and Eugene S. Kryachko.
- Pages: 3219-3220
- First Published: 16 June 2004
A Brighter Future for Protein Design†
- Pages: 3222-3223
- First Published: 15 June 2004
A deeper understanding of the sequence–structure relationship in proteins has resulted from research in the field of protein design. Recent success in designing a protein with a topology not yet observed in nature (see picture) increases our confidence in computational biology and should open the road to the engineering of molecular machines with specified properties and functions.
Asymmetric Catalysis in the Pharmaceutical Industry
- Pages: 3224-3228
- First Published: 16 June 2004

The best catalyst from a chemical perspective is not necessarily the best catalyst from an overall economic perspective. In competing approaches to candoxatril the most selective catalyst for the asymmetric hydrogenation (see scheme) was not the one ultimately chosen. The constraints of the pharmaceutical industry are discussed with regard to the application of asymmetric catalysis to the large-scale synthesis of drug candidates and commercial drugs.
Total Syntheses of Colchicine in Comparison: A Journey through 50 Years of Synthetic Organic Chemistry
- Pages: 3230-3256
- First Published: 15 June 2004
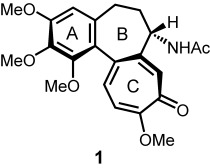
The tubulin-binding alkaloid colchicine (1), isolated from the meadow saffron, is an antimitotic agent with a great pharmaceutical potential. Only a few other natural products have proved to be such a challenge to synthetic chemists for half a century. The comparative analysis of the often fascinating synthetic strategies reveals why this seemingly simple structure still belongs to the “difficult targets”.
Highly Diastereo- and Enantioselective Reactions of Enecarbamates with Ethyl Glyoxylate To Give Optically Active syn and anti α-Alkyl-β-Hydroxy Imines and Ketones†
- Pages: 3258-3260
- First Published: 15 June 2004
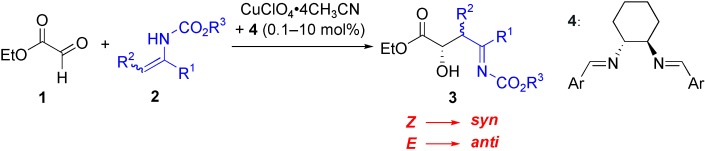
The remarkably selective addition of enecarbamates 2 to ethyl glyoxylate (1) in the presence of a copper–diimine catalyst (0.1 mol %) gives the corresponding imines 3 in high yields with excellent enantioselectivities. A concerted aza-ene-type reaction mechanism was proposed to explain the stereochemical outcome.
Controlled Submolecular Translational Motion in Synthesis: A Mechanically Interlocking Auxiliary†
- Pages: 3260-3264
- First Published: 16 June 2004
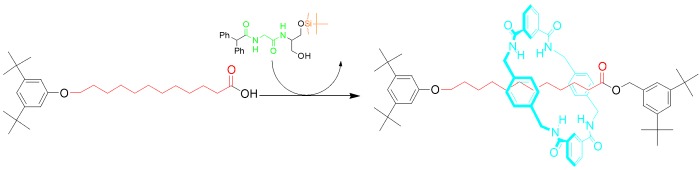
Sew simple: How can you put a molecular bead on a thread when no recognition elements exist between them? A mechanically interlocking auxiliary assembles the macrocycle around a template, controlled submolecular translation moves the ring over the desired substrate and, finally, cleavage of the auxiliary leaves an apparently “impossible” rotaxane (see scheme).
Aromatic Donor–Acceptor Charge-Transfer and Metal-Ion-Complexation-Assisted Folding of a Synthetic Polymer†
- Pages: 3264-3268
- First Published: 16 June 2004
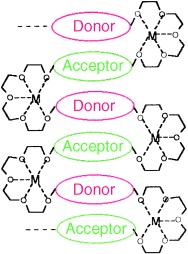
Designer folds: The interplay of three design elements (aromatic donor–acceptor charge-transfer complexation, solvophobic effect, and metal-ion complexation) guided the preparation of a folded structure (see picture) in a synthetic macromolecule. Evidence for this is provided by UV/Vis and 1H NMR spectroscopy.
Expanding and Shrinking Porous Modulation Based on Pillared-Layer Coordination Polymers Showing Selective Guest Adsorption†
- Pages: 3269-3272
- First Published: 15 June 2004
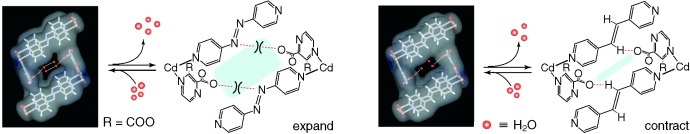
Rationally designed and synthesized, two novel pillar-layered 3D microporous frameworks {[Cd(pzdc)(azpy)]⋅2 H2O}n (1, left) and {[Cd(pzdc)(bpee)]⋅1.5 H2O}n (2, right) have high thermal stability and highly selective adsorption properties. Removal of guest water molecules results in the expansion of 1 but the contraction of 2.
The Exclusivity of Multivalency in Dynamic Covalent Processes†
- Pages: 3273-3278
- First Published: 16 June 2004
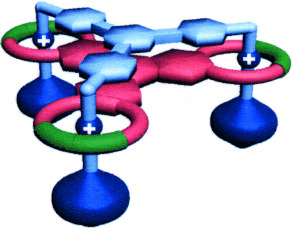
Less is more: It is much less efficient to synthesize both components of a multivalent recognition site separately than it is to use one multivalent component to act as a template for the catalytically orchestrated construction of the other component, as demonstrated by the formation of the mechanically interlocked, triply threaded molecular bundle shown. The situation is reminiscent of nature.
Sol–Gel Reaction Using DNA as a Template: An Attempt Toward Transcription of DNA into Inorganic Materials
- Pages: 3279-3283
- First Published: 16 June 2004
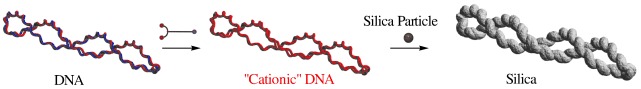
The topologically different structures in plasmid DNA can be successfully transcribed into silica structures by using the DNA as a template for sol–gel polycondensation of tetraethoxysilane after treatment by two cation-exchange steps (see scheme). These findings imply that the different ordered silica structures can be created from the same template through its higher-order conformational changes.
Thermoreversible Gels as Magneto-Optical Switches†
- Pages: 3283-3286
- First Published: 16 June 2004
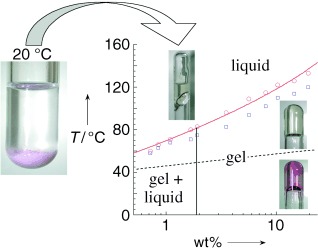
Polyfunctional gels: Thermoreversible switchable gels with tunable magnetic, optical, and rheological properties are obtained from a modified spin-crossover polymeric precursor and alkane solvents (see picture). The iron triazole polymer which acts as the gelator, undergoes a spin crossover that is accompanied by a change in the color of the gel. The transfer of spin-crossover properties from solid materials into gels offers a route to new applications.
Green Oxidation Catalysts: Computational Design of High-Efficiency Models of Galactose Oxidase†
- Pages: 3286-3289
- First Published: 16 June 2004
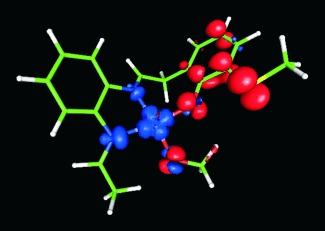
Ab initio calculations were used for the rational design of efficient alcohol oxidation catalysts that mimic the enzyme galactose oxidase. Different ligand substitutions were explored based on natural (copper, depicted) and alternative (rhodium) metal redox centers. The calculated turnover rate for the most efficient copper-based biomimetic compound is greater than that of the natural enzyme.
Inorganic Oxo Compounds React with Alkylating Agents: Implications for DNA Damage†
- Pages: 3290-3292
- First Published: 16 June 2004
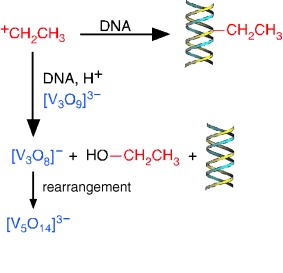
Intercepting Carcinogens: The cancer-preventing properties of inorganic species, such as selenium and vanadium, are well known, but mechanistic understanding is scant. It is shown that inorganic oxo species (e.g., [SeO4]2−, [VO4]3−) can prevent DNA alkylation as well as detoxify alkylating agents by promoting hydrolysis to relatively harmless alcohols (see scheme).
Electrical Contacting of Glucose Oxidase in a Redox-Active Rotaxane Configuration†
- Pages: 3292-3300
- First Published: 16 June 2004
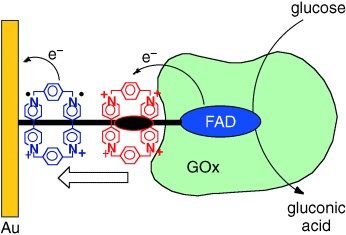
Shuttle of electrons: A redox-active bisbipyridinium cyclophane stoppered by the enzyme glucose oxidase (GOx) in a rotaxane configuration on a molecular wire linked to a gold electrode (see picture) leads to the effective electrical contacting of the biocatalyst that oxidizes glucose at −0.4 V (versus saturated calomel electrode).
A Bis(μ-alkylperoxo)dinickel(II) Complex as a Reaction Intermediate for the Oxidation of the Methyl Groups of the Me2-tpa Ligand to Carboxylate and Alkoxide Ligands†
- Pages: 3300-3303
- First Published: 16 June 2004
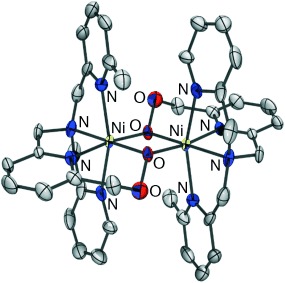
A radical approach: The reaction of [Ni2(OH)2(Me2-tpa)2]2+ with H2O2 resulted in the peroxidation of a methyl group of the Me2-tpa ligand to produce a bis(μ-alkylperoxo)dinickel(II) complex (see ORTEP diagram) as a reaction intermediate for further oxidation to carboxylato and alkoxo complexes [Ni(Me1-tpa-COO)]+ and [Ni2(Me1-tpa-CH2O)2]2+. Me2-tpa=bis[(6-methyl-2-pyridyl)methyl][(2-pyridyl)methyl]amine.
Ruthenium Nanoparticles Supported on Hydroxyapatite as an Efficient and Recyclable Catalyst for cis-Dihydroxylation and Oxidative Cleavage of Alkenes†
- Pages: 3303-3307
- First Published: 16 June 2004
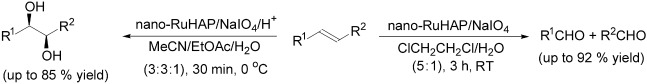
Impregnation of hydroxyapatite with colloidal ruthenium results in the formation of a catalyst that effects cis-dihydroxylation and oxidative cleavage of alkenes to their respective cis-1,2-diols and carbonyl products in good to excellent yields (see scheme). The supported ruthenium catalyst can be easily recycled and reused for consecutive reaction runs without significant deterioration of the catalytic activities. R1, R2=H, alkyl, aryl.
Construction of CS Bonds with a Quaternary Stereocenter through a Formal Michael Reaction: Asymmetric Synthesis of Tertiary Thiols†
- Pages: 3307-3310
- First Published: 16 June 2004
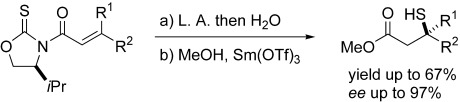
Quaternary stereocenters that bear a sulfur substituent can be created with nearly perfect stereocontrol through an intramolecular Michael-type process. Lewis acids (L.A.) accelerate the intramolecular sulfur-atom transfer from the oxazolidine-2-thione functionality to the β carbon atom of the β,β-disubstituted enoyl moiety, whereas the chirality of the oxazolidine-2-thione portion controls reaction stereochemistry (see scheme).
The Coexistence of Two Different Methane Hydrate Phases under Moderate Pressure and Temperature Conditions: Kinetic versus Thermodynamic Products†
- Pages: 3310-3313
- First Published: 16 June 2004
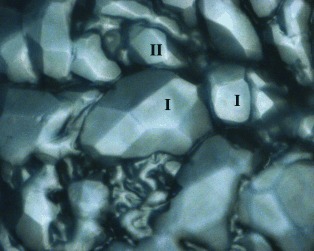
The unexpected coexistence of structure I (s I) and structure II (s II) methane hydrates under conditions where only s I hydrates should be stable (see picture) suggests that the initial product is determined by kinetics. The state of coexistence as well as the transformation of the kinetic product into the thermodynamically stable s I hydrate was documented by microscopic visual observation as well as Raman spectroscopy.
Kinetic Resolution of Amines: A Highly Enantioselective and Chemoselective Acetylating Agent with a Unique Solvent-Induced Reversal of Stereoselectivity†
- Pages: 3314-3317
- First Published: 16 June 2004
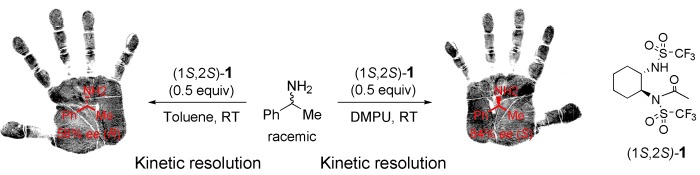
Solvents lend a hand: Changing the polarity of the reaction solvent from 1,3-dimethyltetrahydropyrimidin-2-one (DMPU) to toluene reverses the stereoselectivity observed in the acetylation of amines with (1S,2S)-1 (see scheme). Optimizing the reaction conditions led to an unprecedented 90 % ee (S) in DMPU at −20 °C with a 33 % conversion.
Amplification of Enantiomeric Excess in a Proline-Mediated Reaction†
- Pages: 3317-3321
- First Published: 16 June 2004
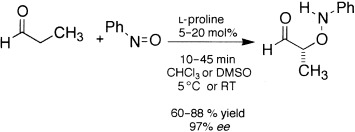
The evolution of homochirality from simple organic molecules can perhaps be modeled on the proline-mediated aminoxylation of aldehydes (see scheme). The observed accelerating reaction rate combined with an amplification of the enantiomeric excess of the product is attributed to an autoinductive reaction mediated by an adduct of proline and the product.
Isolation of [1]Ruthenocenophanes: Synthesis of Polyruthenocenylstannanes by Ring-Opening Polymerization†
- Pages: 3321-3325
- First Published: 16 June 2004
A Biomimetic Route to the Peptide Alkaloid Anachelin†
- Pages: 3327-3329
- First Published: 16 June 2004
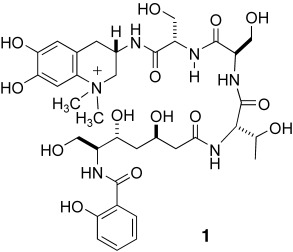
A postulated biogenesis forms the basis for a synthetic route to the natural product anachelin H (1). Key steps include a tellurium-mediated, oxidative aza annulation and a Claisen condensation under mild conditions. Experiments with a model substrate indicate that it is likely that a catechol oxidase-type enzyme is involved in the biosynthesis of the anachelin chromophore.
A LiCl-Mediated Br/Mg Exchange Reaction for the Preparation of Functionalized Aryl- and Heteroarylmagnesium Compounds from Organic Bromides†
- Pages: 3333-3336
- First Published: 15 June 2004

A wide range of aryl and heteroaryl bromides, which are usually sluggish in exchange reactions, are readily converted into the corresponding Grignard reagents by means of a Br/Mg exchange reaction triggered by iPrMgCl⋅LiCl (see scheme). These Grignard intermediates react with electrophiles in good yields.
Generation and Enzymatic Amplification of High-Density Functionalized DNA Double Strands†
- Pages: 3337-3340
- First Published: 15 June 2004
Up to eight modifications could be incorporated into a DNA heteroduplex (see picture) by introducing different sets of 2′-deoxynucleotide derivatives through DNA polymerase mediated primer extension. All four natural nucleobases in each strand were substituted with different base-modified analogues. PCR conditions are described that allow the direct amplification of fully functionalized DNA double strands.





![Isolation of [1]Ruthenocenophanes: Synthesis of Polyruthenocenylstannanes by Ring-Opening Polymerization](/cms/asset/80a5c33e-d93c-4124-8bb9-980225b77cf0/mcontent.jpg)