Construction of CS Bonds with a Quaternary Stereocenter through a Formal Michael Reaction: Asymmetric Synthesis of Tertiary Thiols†
Claudio Palomo Prof. Dr.
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorMikel Oiarbide Prof. Dr.
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorFlavia Dias
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorRosa López Dr.
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorAnthony Linden Dr.
Organisch-chemisches Institut, Universität Zürich, Winterthurerstrasse 190, 8057 Zürich, Switzerland
crystal structure analysis
Search for more papers by this authorClaudio Palomo Prof. Dr.
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorMikel Oiarbide Prof. Dr.
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorFlavia Dias
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorRosa López Dr.
Departamento de Química Orgánica I, Facultad de Química, Universidad del País Vasco, Apdo. 1072, 20080 San Sebastián, Spain, Fax: (+34) 943-212-236
Search for more papers by this authorAnthony Linden Dr.
Organisch-chemisches Institut, Universität Zürich, Winterthurerstrasse 190, 8057 Zürich, Switzerland
crystal structure analysis
Search for more papers by this authorWe thank The University of the Basque Country (EHU/UPV) and Ministerio de Ciencia y Tecnología (Spain) for financial support. A Ramón y Cajal grant to R.L. from Ministerio de Educación Cultura y Deporte and a predoctoral grant to F.D. from EHU/UPV are acknowledged.
Graphical Abstract
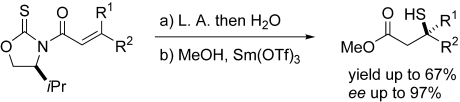
Quaternary stereocenters that bear a sulfur substituent can be created with nearly perfect stereocontrol through an intramolecular Michael-type process. Lewis acids (L.A.) accelerate the intramolecular sulfur-atom transfer from the oxazolidine-2-thione functionality to the β carbon atom of the β,β-disubstituted enoyl moiety, whereas the chirality of the oxazolidine-2-thione portion controls reaction stereochemistry (see scheme).
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53889_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For an updated account on the synthesis of thiols, sulfides, and derivatives, see: D. J. Procter, J. Chem. Soc. Perkin Trans. 1 2001, 335–354 and previous review articles in the series.
- 2For a review on metal-catalyzed CS bond formation, see: T. Kondo, T. Mitsudo, Chem. Rev. 2000, 100, 3205–3220.
- 3P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis, Pergamon, Oxford, 1992.
- 4For recent examples, see:
- 4aD. F. Taber, G. J. Gorski, L. M. Liable-Sands, A. L. Rheingold, Tetrahedron Lett. 1997, 38, 6317–6318;
- 4bO. Miyata, T. Shinada, I. Ninomiya, T. Naito, Tetrahedron 1997, 53, 2421–2438;
- 4cC.-H. Lin, K.-S. Yang, J.-P. Pan, K. Chen, Tetrahedron Lett. 2000, 41, 6815–6819;
- 4dM. Node, K. Nishide, Y. Shigeta, H. Shiraki, K. Obata, J. Am. Chem. Soc. 2000, 122, 1927–1936;
- 4eK. Nishide, S.-i. Ohsugi, H. Shiraki, H. Tamakita, M. Node, Org. Lett. 2001, 3, 3121–3124.
- 5For some recent examples, see:
- 5aE. Emori, T. Arai, H. Sasai, M. Shibasaki, J. Am. Chem. Soc. 1998, 120, 4043–4044;
- 5bM. Saito, M. Nakajima, S. Hashimoto, Tetrahedron 2000, 56, 9589–9594;
- 5cV. A. Castelli, A. D. Cort, L. Mandolini, D. N. Reinhoudt, D. L. Schiaffino, Chem. Eur. J. 2000, 6, 1193–1198;
10.1002/(SICI)1521-3765(20000403)6:7<1193::AID-CHEM1193>3.3.CO;2-6 PubMed Web of Science® Google Scholar
- 5dM. Saito, M. Nakajima, S. Hashimoto, Chem. Commun. 2000, 1851–1852;
- 5eS. Kobayashi, C. Ogawa, M. Kawamura, M. Sugiura, Synlett 2001, 983–985;
- 5fP. McDaid, Y. Chen, L. Deng, Angew. Chem. 2002, 114, 348–350;
10.1002/1521-3757(20020118)114:2<348::AID-ANGE348>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 2002, 41, 338–340;10.1002/1521-3773(20020118)41:2<338::AID-ANIE338>3.0.CO;2-M CAS PubMed Web of Science® Google Scholar
- 5gK. Nishimura, K. Tomioka, J. Org. Chem. 2002, 67, 431–434;
- 5hN. Prabagaran, G. Sundararajan, Tetrahedron: Asymmetry 2002, 13, 1053–1058.
- 6Reviews on catalytic enantioselective Michael additions:
- 6aN. Krause, A. Hoffmann-Röder, Synthesis 2001, 171–196;
- 6bM. P. Sibi, S. Manyem, Tetrahedron 2000, 56, 8033–8061.
- 7For the synthesis of quaternary CS systems other than thiols asymmetrically, see: Sulfoxides:
- 7aD. A. Evans, G. C. Andews, Acc. Chem. Res. 1974, 7, 147–155; sulfones from rearrangement of chiral sulfinates:
- 7bK. Hiroi, M. Yamamoto, Y. Kurihara, H. Yonezawa, Tetrahedron Lett. 1990, 31, 2619–2622; sulfides from rearrangement of sulfur ylides:
- 7cX. Zhang, Z. Qu, Z. Ma, W. Shi, X. Jin, J. Wang, J. Org. Chem. 2002, 67, 5621–5625; sulfides from rearrangement of allyl xanthates:
- 7dM. S. Chambers, E. J. Thomas, D. J. Williams, J. Chem. Soc. Chem. Commun. 1987, 1228–1230.
- 8The preparation of a tertiary arylsulfide is described in reference [5a] (53 % yield and 85 % ee); another one in reference [5b] (43 % yield and 10 % ee).
- 9M. E. Jung in Comprehensive Organic Synthesis, Vol. 4 (Eds.: ), Pergamon, Oxford, 1991, p. 17.
- 10
- 10aJ. A. Marshall, S. L. Crooks, B. S. DeHoff, J. Org. Chem. 1988, 53, 1616–1623;
- 10bJ. A. Marshall, M. W. Andersen, J. Org. Chem. 1992, 57, 2766–2768.
- 11Reviews:
- 11aK. Fuji, Chem. Rev. 1993, 93, 2037–2066;
- 11bE. J. Corey, A. Guzman-Perez, Angew. Chem. 1998, 110, 402–415;
10.1002/(SICI)1521-3757(19980216)110:4<402::AID-ANGE402>3.0.CO;2-6 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 388–401;10.1002/(SICI)1521-3773(19980302)37:4<388::AID-ANIE388>3.0.CO;2-V PubMed Web of Science® Google Scholar
- 11cJ. Christoffers, A. Mann, Angew. Chem. 2001, 113, 4725–4732;
10.1002/1521-3757(20011217)113:24<4725::AID-ANGE4725>3.0.CO;2-L Google ScholarAngew. Chem. Int. Ed. 2001, 40, 4591–4597;10.1002/1521-3773(20011217)40:24<4591::AID-ANIE4591>3.0.CO;2-V CAS PubMed Web of Science® Google Scholar
- 11dI. Denissova, L. Barriault, Tetrahedron 2003, 59, 10 105–10 146.
- 12The use of Michael addition reactions in the construction of quaternary stereocenters: J. Christoffers, A. Baro, Angew. Chem. 2003, 115, 1726–1728;
10.1002/ange.200201614 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1688–1690.
- 13C. Palomo, M. Oiarbide, F. Dias, A. Ortiz, A. Linden, J. Am. Chem. Soc. 2001, 123, 5602–5603.
- 14For further development by others, see: T. Kataoka, H. Kinoshita, S. Kinoshita, T. Osamura, S. Watanabe, T. Iwamura, O. Muraoka, G. Tanabe, Angew. Chem. 2003, 115, 2995–2997;
10.1002/ange.200351106 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2889–2891.
- 15For Lewis acid-assisted cyclization of N-enoyl thioureas leading to 1,3-thiazines, see: M. Dzurilla, P. Kutschy, P. Kristan, Synthesis 1985, 933–934.
- 16While the treatment with SnCl2 led to the recovery of unreacted 3, the reaction with other Lewis acids such as TiCl4, Sm(OTf)3, AlCl3, AlEt2Cl, BCl3, and BBr3 led to variable quantities of undesired side products.
- 17CCDC-228804 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or [email protected]).
- 18Mixtures of different E/Z composition were obtained by either column chromatography- or recrystallization-driven enrichment of the original E/Z mixture. See Supporting Information for details.
- 19For example, it has been described recently that the conjugate addition of lithium thiophenolate to an E and Z mixture of a cyclic enone gives exclusively one diastereomeric addition product. T. J. Houghton, C. Soongyu, V. H. Rawal, Org. Lett. 2001, 3, 3615–3617.
- 20Note for this case it is the opposite configuration of both the auxiliary and the resulting tertiary sulfanyl. Also, see reference [25].
- 21The synthesis of β,β-disubstituted Michael acceptors with either solely E or solely Z configuration is, in general, not so straightforward. For further information, see, for instance, N. Zhu, D. G. Hall, J. Org. Chem. 2003, 68, 6066–6069, and references therein.
- 22There are two possible rationales: kinetic production of the observed diastereomer through any reaction pathway involving a common intermediate reachable from both E- and Z-configured enoyl derivatives, and thermodynamic equilibration of the formed diastereomeric products before final hydrolysis. Among the possible pathways for the first rationale would be the virtual E/Z isomerization of the substrate under the reaction conditions. After analysis by NMR spectroscopy of aliquots taken for a set of reactions at times corresponding to different degrees of reaction conversion, however, no detectable E/Z isomerization could be observed, and this possibility can be ruled out.
- 23
- 23aE. Lee, E. J. Jeong, E. J. Kang, L. T. Sung, S. K. Hong, J. Am. Chem. Soc. 2001, 123, 10 131–10 132;
- 23bA. Ortiz, L. Quintero, H. Hernandez, S. Maldonado, G. Mendoza, S. Bernés, Tetrahedron Lett. 2003, 44, 1129–1132.
- 24M. Prashad, D. Har, H.-Y. Kim, O. Repic, Tetrahedron Lett. 1998, 39, 7067–7070.
- 25The enantiomer of 10 is obtained in ≥97 % ee from the reduction of 7 under otherwise identical conditions.