The Exclusivity of Multivalency in Dynamic Covalent Processes†
Jovica D. Badjić Dr.
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorStuart J. Cantrill Dr.
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorRobert H. Grubbs Prof.
Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorErin N. Guidry
Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorRaul Orenes
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorJ. Fraser Stoddart Prof.
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorJovica D. Badjić Dr.
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorStuart J. Cantrill Dr.
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorRobert H. Grubbs Prof.
Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorErin N. Guidry
Arnold and Mabel Beckman Laboratory of Chemical Synthesis, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorRaul Orenes
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorJ. Fraser Stoddart Prof.
California NanoSystems Institute and Department of Chemistry and Biochemistry, University of California, Los Angeles, 405 Hilgard Avenue, Los Angeles, CA 90095, USA, Fax: (+1) 310-206-1843
Search for more papers by this authorWe thank the National Science Foundation (CHE 0317170) for funding this research which is also supported in part by equipment grants (CHE 9974928 and CHE 0092036) from the National Science Foundation. We also thank the Office of Naval Research for support through its MURI program.
Graphical Abstract
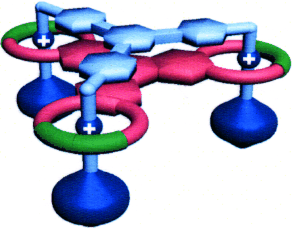
Less is more: It is much less efficient to synthesize both components of a multivalent recognition site separately than it is to use one multivalent component to act as a template for the catalytically orchestrated construction of the other component, as demonstrated by the formation of the mechanically interlocked, triply threaded molecular bundle shown. The situation is reminiscent of nature.
References
- 1
- 1aR. T. Lee, Y. C. Lee, Glycoconjugate J. 2000, 17, 543–551;
- 1bJ. J. Lundquist, E. J. Toone, Chem. Rev. 2002, 102, 555–578;
- 1cS. L. Tobey, E. V. Anslyn, J. Am. Chem. Soc. 2003, 125, 10 963–10 970;
- 1dG. Ercolani, J. Am. Chem. Soc. 2003, 125, 16 097–16 103.
- 2With a few provisos, the adage “many a mickle makes a muckle”, comes to mind; for some key articles relating the multivalency effects, see:
- 2aE. J. Gordon, W. J. Sanders, L. L. Kiessling, Nature 1998, 392, 30–31;
- 2bJ. Rao, J. Lahiri, L. Isaacs, R. M. Weiss, G. M. Whitesides, Science 1998, 280, 708–711;
- 2cP. I. Kitov, J. M. Sadawska, G. Mulvey, G. D. Armstrong, H. Ling, N. S. Pannu, R. J. Read, D. R. Bundle, Nature 2000, 403, 669–672;
- 2dJ. E. Gestwicki, C. W. Cairo, L. E. Strong, K. A. Oetjen, L. L. Kiessling, J. Am. Chem. Soc. 2002, 124, 14 922–14 933;
- 2eE. K. Woller, E. D. Walter, J. R. Morgan, D. J. Singel, M. J. Cloninger, J. Am. Chem. Soc. 2003, 125, 8820–8826.
- 3
- 3aR. T. Lee, Y. C. Lee, Acc. Chem. Res. 1995, 28, 321–327;
- 3bK. Drickamer, Nat. Struct. Biol. 1995, 2, 437–439;
- 3cJ. M. Rini, Annu. Rev. Biophys. Biomol. Struct. 1995, 24, 551–557;
- 3dR. Roy, Polym. News 1996, 21, 226–232;
- 3eW. I. Weiss, K. Drickamer, Annu. Rev. Biochem. 1996, 65, 441–473;
- 3fN. Jayaraman, S. A. Nepogodiev, J. F. Stoddart, Chem. Eur. J. 1997, 3, 1193–1199;
- 3gL. L. Kiessling, J. E. Gestwicki, L. E. Strong, Curr. Opin. Chem. Biol. 2000, 4, 696–703;
- 3hN. Rockendorf, T. H. Lindhorst, Top. Curr. Chem. 2001, 217, 201–238;
- 3iW. B. Turnbull, J. F. Stoddart, Rev. Mol. Biotechnol. 2002, 90, 231–255;
- 3jM. Dubber, J. M. J. Fréchet, Bioconjugate Chem. 2003, 14, 239–246.
- 4P. I. Kitov, D. R. Bundle, J. Am. Chem. Soc. 2003, 125, 16 271–16 284.
- 5T. Christensen, D. M. Gooden, J. E. Kung, E. J. Toone, J. Am. Chem. Soc. 2003, 125, 7357–7366.
- 6A recent study carried out on the high-avidity, reversible low-affinity multivalent interactions of the lectin XL35 with the jelly coat proteins surrounding oocytes in Xenopus laevis shows that they require a very long time to reach equilibrium, so long in fact that the partners do not attain true equilibrium on the timescale of the biological event, namely polyspermy, yet are such that their metastable mode of interaction is probably more than enough to guarantee an insurmountable physical block to polyspermy; see
- 6aE. Arranz-Plaza, A. S. Tracy, A. Siriwardena, J. M. Pierce, G.-J. Boons, J. Am. Chem. Soc. 2002, 124, 13 035–13 046; in the strict self-assembly of a triply threaded two-component superbundle, starting from a tritopic receptor in which three benzo[24]crown-8 macrorings are fused onto a triphenylene core and a trifurcated trication wherein three bipyridinium units are linked 1,3,5 to a central benzenoid core, it transpires that the rapid formation of a doubly threaded two-component complex is followed by an extremely slow conversion (a week at 253 K in CD3COCD3 to reach equilibrium) of this kinetically controlled product into a thermodynamically controlled one—namely, a triply threaded two-component superbundle; see:
- 6bJ. D. Badjić, S. J. Cantrill, J. F. Stoddart, J. Am. Chem. Soc. 2004, 126, 2288–2289.
- 7
- 7aP. A. Brady, R. P. Bonar-Law, S. J. Rowan, C. J. Suckling, J. K. M. Sanders, Chem. Commun. 1996, 319–320;
- 7bP. A. Brady, J. K. M. Sanders, Chem. Soc. Rev. 1997, 26, 327–336;
- 7cJ.-M. Lehn, Chem. Eur. J. 1999, 5, 2455–2463;
10.1002/(SICI)1521-3765(19990903)5:9<2455::AID-CHEM2455>3.0.CO;2-H CAS Web of Science® Google Scholar
- 7dJ. K. M. Sanders, Pure Appl. Chem. 2000, 72, 2265–2274;
- 7eL. M. Greig, D. Philp, Chem. Soc. Rev. 2001, 30, 287–302;
- 7fS. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. 2002, 114, 938–993;
10.1002/1521-3757(20020315)114:6<938::AID-ANGE938>3.0.CO;2-K Google ScholarAngew. Chem. Int. Ed. 2002, 41, 899–952.10.1002/1521-3773(20020503)41:9<1460::AID-ANIE11111460>3.0.CO;2-N CAS Web of Science® Google Scholar
- 8
- 8aD. A. Fulton, S. J. Cantrill, J. F. Stoddart, J. Org. Chem. 2002, 67, 7968–7981;
- 8bJ. N. Lowe, D. A. Fulton, S.-H. Chiu, A. M. Elizarov, S. J. Cantrill, S. J. Rowan, J. F. Stoddart, J. Org. Chem., in press; see also:
- 8cH. W. Gibson, N. Yamaguchi, L. Hamilton, J. W. Jones, J. Am. Chem. Soc. 2002, 124, 4653–4665.
- 9
- 9aM. C. T. Fyfe, J. N. Lowe, J. F. Stoddart, D. J. Williams, Org. Lett. 2000, 2, 1221–1224;
- 9bV. Balzani, M. Clemente-Leon, A. Credi, J. N. Lowe, J. D. Badjić, J. F. Stoddart, D. J. Williams, Chem. Eur. J. 2003, 9, 5348–5360.
- 10J. D. Badjić, V. Balzani, A. Credi, J. N. Lowe, S. Silvi, J. F. Stoddart, Chem. Eur. J. 2004, 10, 1926–1935.
- 11This prototype has been developed yet further in the incrementally staged-design, bottom-up construction, extensive characterization, and chemically driven operation of a two-component molecular machine that behaves like a nanoscale elevator; see: J. D. Badjić, V. Balzani, A. Credi, S. Silvi, J. F. Stoddart, Science 2004, 303, 1845–1849.
- 12
- 12aV. Balzani, A. Credi, F. M. Raymo, J. F. Stoddart, Angew. Chem. 2000, 112, 3484–3530;
10.1002/1521-3757(20001002)112:19<3484::AID-ANGE3484>3.0.CO;2-O Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3348–3391;10.1002/1521-3773(20001002)39:19<3348::AID-ANIE3348>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 12bV. Balzani, A. Credi, M. Venturi in Molecular Devices and Machines—A Journey into the Nano World, Wiley-VCH, Weinheim, 2003.
10.1002/3527601600 Google Scholar
- 13A recent communication of dynamic chiral crown ether complexes during cyclic acetal formation lists a selection of the various reaction types which can be carried out under thermodynamic control, to create dynamic combinatorial libraries, from which selected products can often be amplified with a specific template; see: B. Fuchs, A. Nelson, A. Star, J. F. Stoddart, S. Vidal, Angew. Chem. 2003, 115, 4352–4356; Angew. Chem. Int. Ed. 2003, 42, 4220–4224.
- 14T. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18–29.
- 15For an example of a [2]catenane synthesis employing RORCM and operating under thermodynamic control, see:
- 15aT. J. Kidd, D. A. Leigh, A. J. Wilson, J. Am. Chem. Soc. 1999, 121, 1599–1600; for examples of [2]catenanes utilizing RCM, see:
- 15bD. G. Hamilton, N. Feeder, S. J. Teat, J. K. M. Sanders, New J. Chem. 1998, 22, 1019–1021;
- 15cM. Weck, B. Mohr, J.-P. Sauvage, R. H. Grubbs, J. Org. Chem. 1999, 64, 5463–5471;
- 15dP. Mobian, J- M. Kern, J.-P. Sauvage, J. Am. Chem. Soc. 2003, 125, 2016–2017; for examples of [2]rotaxane syntheses using RCM, see:
- 15eJ. A. Wisner, P. D. Beer, M. G. B. Drew, M. R. Sambrook, J. Am. Chem. Soc. 2002, 124, 12 469–12 476;
- 15fJ. S. Hannam, T. J. Kidd, D. A. Leigh, A. J. Wilson, Org. Lett. 2003, 5, 1907–1910;
- 15gR. G. E. Coumans, J. A. A. W. Elemans, P. Thordarson, R. J. M. Nolte, A. E. Rowan, Angew. Chem. 2003, 115, 674–678;
10.1002/ange.200390147 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 650–653.
- 16Olefin metathesis, catalyzed by RuII complexes developed at CALTECH, have found wide application in, for example, the reversible covalent capture of supramolecular assemblies; see:
- 16aT. D. Clark, K. Kobayashi, M. R. Ghadiri, Chem. Eur. J. 1999, 5, 782–792;
- 16bF. Cardullo, M. Crego Calama, B. H. M. Snellink-Ruël, J.-L. Weidmann, A. Bielejewska, R. Fokkens, N. M. M. Nibbering, P. Timmerman, D. N. Reinhoudt, Chem. Commun. 2000, 367–368;
- 16cM. O. Vysotsky, M. Bolte, I. Thondorf, V. Böhmer, Chem. Eur. J. 2003, 9, 3375–3382; for the cross-linking of dendrimer hosts employed as monomolecular imprinting systems, see:
- 16dE. Mertz, S. C. Zimmerman, J. Am. Chem. Soc. 2003, 125, 3424–3425.
- 17A. F. M. Kilbinger, S. J. Cantrill, A. W. Waltman, M. W. Day, R. H. Grubbs, Angew. Chem. 2003, 115, 3403–3407; Angew. Chem. Int. Ed. 2003, 42, 3281–3285.
- 18H. Iwamoto, K. Itoh, H. Nagamiya, Y. Fukuzawa, Tetrahedron Lett. 2003, 44, 5773–5776.
- 19M. C. T. Fyfe, J. F. Stoddart, Acc. Chem. Res. 1997, 30, 393–401.
- 20
- 20aA. G. Kolchinski, D. H. Busch, N. W. Alcock, J. Chem. Soc. Chem. Commun. 1995, 1289–1291;
- 20bP. R. Ashton, P. J. Campbell, E. J. T. Chrystal, P. T. Glink, S. Menzer, D. Philp, N. Spencer, J. F. Stoddart, P. A. Tasker, D. J. Williams, Angew. Chem. 1995, 107, 1997–2001;
10.1002/ange.19951071711 Google ScholarAngew. Chem. Int. Ed. Engl. 1995, 34, 1865–1869;
- 20cP. R. Ashton, E. J. T. Chrystal, P. T. Glink, S. Menzer, C. Schiavo, N. Spencer, J. F. Stoddart, P. A. Tasker, A. J. P. White, D. J. Williams, Chem. Eur. J. 1996, 2, 709–728.
- 21When the reaction is followed by 1H NMR spectroscopy, one major product, namely [6-H3][PF6]3, is formed with high conversion (>95 %) from the starting materials, namely, 3 and [5-H3][PF6]3. The yield of 62 % recorded in the Experimental Section is considerably lower because of the loss of [6-H3][PF6]3 during silica gel chromatography.
- 22IMesH2=1,3-dimesityl-4,5-dihydroimidazol-2-ylidene; see: M. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953–956.