Journal list menu
Export Citations
Download PDFs
Table of Contents
Olefin-Metathesis Catalysts for the Preparation of Molecules and Materials (Nobel Lecture)†
- First Published: 24 May 2006
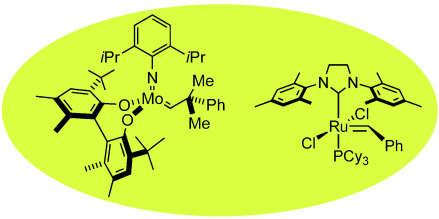
Metathesis reactions are among the most important processes in organic synthesis. The decisive breakthrough in making these reactions practical for industrial purposes, which range from the synthesis of polymers to pharmaceuticals, came with the discovery of the reaction mechanism by Yves Chauvin and the targeted development of transition-metal-based metathesis catalysts by Richard Schrock and Robert Grubbs. The winners of the Chemistry Nobel Prize in 2005 present first-hand accounts of these developments.
A Series of Well-Defined Metathesis Catalysts–Synthesis of [RuCl2(CHR′)(PR3)2] and Its Reactions†
- First Published: October 2, 1995
![A Series of Well-Defined Metathesis Catalysts–Synthesis of [RuCl2(CHR′)(PR3)2] and Its Reactions](/cms/asset/e6272d1e-47a8-4205-a803-64f673df9afa/must001.jpg)
Substantially better initiators and therefore considerably more reactive than complexes of type A, the novel metathesis catalysts B are easily prepared by the reaction of [RuCl2(PPh3)3] with the appropriate diazoalkanes and subsequent phosphane exchange. While the PPh3 derivatives are excellent catalysts for living ROMP of norbornene, the complex with R = Cy, R′ = Ph is also a very efficient catalyst for the metathesis of acyclic alkenes.
Highly Efficient Synthesis of Covalently Cross-Linked Peptide Helices by Ring-Closing Metathesis
- First Published: 23 December 1998
Influence of Perfluoroarene–Arene Interactions on the Phase Behavior of Liquid Crystalline and Polymeric Materials
- First Published: 15 September 1999
Highly Efficient Ring-Opening Metathesis Polymerization (ROMP) Using New Ruthenium Catalysts Containing N-Heterocyclic Carbene Ligands
- First Published: 11 August 2000
Up to one hundred thousand equivalents of a variety of low-strain cyclic olefins, such as cyclooctadiene, cyclooctene, and several functionalized and sterically hindered derivatives, were polymerized by using highly active ruthenium-based ring-opening metathesis polymerization (ROMP) catalysts [Eq. (1)]. Efficient syntheses of other polymeric structures were also accomplished.
Ruthenium-Based Four-Coordinate Olefin Metathesis Catalysts
- First Published: 26 September 2000
A series of four-coordinate RuII alkylidenes (see scheme for an example) has been prepared as analogues of the proposed olefin metathesis intermediate [(PCy3)Cl2Ru=CHPh]. These complexes exhibit unusual trigonal-pyramidal solid-state geometries, and are rendered highly active for ring-closing metathesis by the addition of HCl.
Highly Active Metathesis Catalysts Generated In Situ from Inexpensive and Air-Stable Precursors
- First Published: 04 January 2001
Ruthenium vinylidenes: metathesis catalysis on the bench? Ruthenium vinylidene compounds 1, generated in situ from readily available, air-stable precursors, are used to catalyze ring-closing metathesis, ene–yne metathesis, cross-metathesis, and ring-opening metathesis polymerization. Bulky imidazolylidene ligands and terminal alkynes were found to be necessary components for achieving high catalytic activity.
Olefin Metathesis with 1,1-Difluoroethylene
- First Published: 14 September 2001
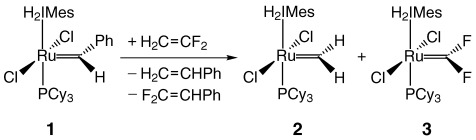
Unsaturated halocarbene complexes can be synthesized in the first example of olefin metathesis with a directly fluorinated substrate. The ruthenium catalyst 1 reacts with 1,1-difluoroethylene to yield the corresponding methylidene [Ru]=CH2 (2) and difluorocarbene [Ru]=CF2 complexes (3). H2IMes=1,3-dimesityl-4,5-dihydroimidazol-2-ylidene; Cy=cyclohexyl.
A One-Pot Cross-Metathesis/Allylboration Reaction: A Three-Component Coupling for the Synthesis of Functionalized Homoallylic Alcohols
- First Published: 07 March 2002
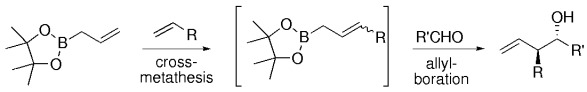
Reactive reagents can be prepared by means of olefin cross-metathesis. A wide variety of functionalized allyl boronates were synthesized and were found to react cleanly with aldehydes to afford homoallylic alcohols, without prior purification (see scheme). Olefins that bear allylic ethers, halides, protected aldehydes, and sterically encumbering groups are viable substrates for this reaction.
Arene–Perfluoroarene Interactions as Physical Cross-Links for Hydrogel Formation
- First Published: 02 May 2002
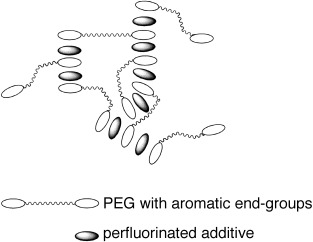
Stiff PEGs: Aqueous solutions of pyrene end-capped poly(ethylene glycol)s (PEGs) increase their viscosity in the presence of octafluoronaphthalene possibly as a result of aggregation by face-to-face stacking (shown schematically). The exploitation of the arene–perfluoroarene supramolecular synthon in solution is reported.
Formal Vinyl CH Activation and Allylic Oxidation by Olefin Metathesis
- First Published: 30 August 2002
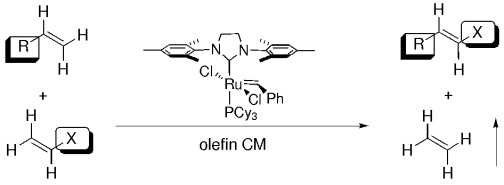
Stereoselective and chemoselective olefin cross metathesis can be viewed as a highly selective and efficient set of reactions that provide the same products as would selective CH activation and allylic oxidation (see scheme for an example). More active catalyst systems will provide an efficient process to functionalized products from readily available olefins. Cy=cyclohexyl.
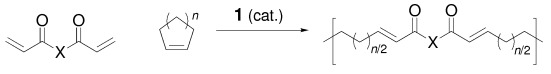
Synthesis of A,B-Alternating Copolymers by Ring-Opening-Insertion-Metathesis Polymerization†
- First Published: 18 October 2002
A Practical and Highly Active Ruthenium-Based Catalyst that Effects the Cross Metathesis of Acrylonitrile†
- First Published: 31 October 2002
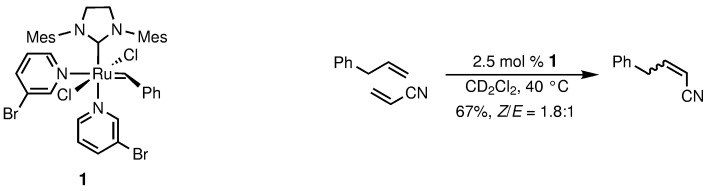
The highest initiation rate of any reported ruthenium-based catalyst was found for the new olefin-metathesis catalyst [(H2IMes)(3-Br-py)2(Cl)2RuCHPh] (1), which was synthesized in one step from commercially available reagents. Complex 1 is highly efficient for the cross metathesis of acrylonitrile, which is generally a poor substrate for metathesis reactions (e.g., see scheme). Mes=2,4,6-trimethylphenyl.
Controlled Living Ring-Opening-Metathesis Polymerization by a Fast-Initiating Ruthenium Catalyst†
- First Published: 16 April 2003
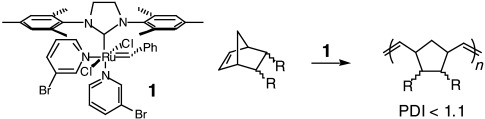
Highly active with ultrafast initiation, the catalyst 1 promotes the controlled, living, ring-opening polymerization of various norbornene and 7-oxonorbornene derivatives (see scheme). The high ki/kp ratio associated with the polymerization reactions results in better molecular-weight control and narrower polydispersity indexes (PDIs, ki=rate constant for initiation, kp=rate constant for polymerization).
Magic Ring Rotaxanes by Olefin Metathesis†
- First Published: 16 July 2003
Trick or treat? Ruthenium alkylidene catalyzed ring-closing metathesis of crown ether like diene substrates around a dumbbell-shaped secondary ammonium ion affords [2]rotaxanes. The reversible nature of this process has been demonstrated through a “magic ring” synthesis, wherein the preformed olefinic macrocycle and dumbbell-shaped component equilibrate to form the hydrogen-bond-stabilized [2]rotaxane in the presence of a metathesis catalyst (see scheme).
The Exclusivity of Multivalency in Dynamic Covalent Processes†
- First Published: 16 June 2004
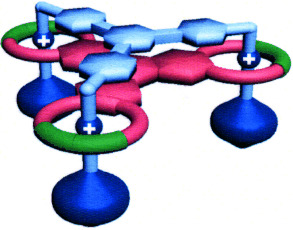
Less is more: It is much less efficient to synthesize both components of a multivalent recognition site separately than it is to use one multivalent component to act as a template for the catalytically orchestrated construction of the other component, as demonstrated by the formation of the mechanically interlocked, triply threaded molecular bundle shown. The situation is reminiscent of nature.
Highly Active Chiral Ruthenium Catalysts for Asymmetric Cross- and Ring-Opening Cross-Metathesis†
- First Published: 14 November 2006
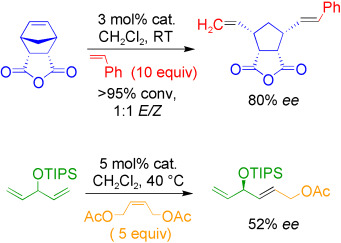
Metathesis takes sides: The scope of asymmetric metathesis has been expanded with the use of chiral ruthenium catalysts for asymmetric ring-opening cross-metathesis and for the first example of an asymmetric cross-metathesis (see scheme, TIPS=triisopropylsilyl). Information about the mechanism of asymmetric ring-opening cross-metathesis should allow the development of more selective catalysts.
Monofunctional Metathesis Polymers via Sacrificial Diblock Copolymers†
- First Published: 27 November 2006
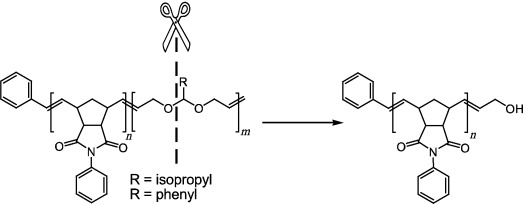
A small price to pay: The second block of a diblock copolymer is “sacrificed” in order to leave behind a monofunctionalized metathesis polymer with a hydroxy end group. By incorporation of a dioxepine unit into the copolymer, a breaking point is created between the block to be end-functionalized and the block to be sacrificed.
Small-Molecule N-Heterocyclic-Carbene-Containing Olefin-Metathesis Catalysts for Use in Water†
- First Published: 22 June 2007
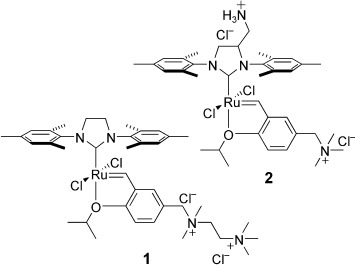
ROMPing around in water: Two well-defined, small-molecule olefin-metathesis catalysts (1 and 2) are introduced. While they are insufficiently stable to mediate most cross-metathesis reactions in water, these catalysts competently mediate ring-opening metathesis polymerization (ROMP) and ring-closing metathesis reactions in an aqueous environment.
Double CH Activation of an N-Heterocyclic Carbene Ligand in a Ruthenium Olefin Metathesis Catalyst†
- First Published: 22 June 2007
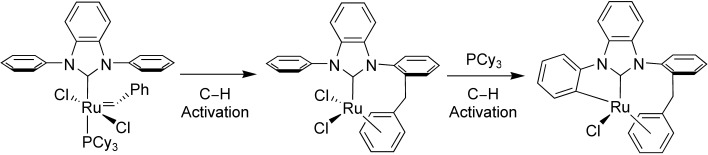
Having a breakdown: Decomposition of the olefin metathesis catalyst [(biph)(PCy3)Cl2RuC(H)Ph] (biph= N,N′-diphenylbenzimidazol-2-ylidene, Cy=cyclohexyl) results in benzylidene insertion into an ortho CH bond of an N-phenyl group of the biph ligand. The ruthenium center further inserts into another ortho CH bond of the other N-phenyl ring to give a new RuC bond as a part of a five-membered metallacycle (see scheme).




 Robert H. Grubbs (California Institute of Technology), who recently celebrated his 75th birthday, shared the 2005 Nobel Prize in Chemistry with Richard R. Schrock and Yves Chauvin. Grubbs is renowned for the development of a series of ruthenium-containing catalysts for olefin metathesis, which have applications ranging from organic synthesis to supramoleuclar and polymer chemistry. In this virtual issue, we feature his Nobel Lecture as well as a selection of his papers published in Angewandte Chemie.
Robert H. Grubbs (California Institute of Technology), who recently celebrated his 75th birthday, shared the 2005 Nobel Prize in Chemistry with Richard R. Schrock and Yves Chauvin. Grubbs is renowned for the development of a series of ruthenium-containing catalysts for olefin metathesis, which have applications ranging from organic synthesis to supramoleuclar and polymer chemistry. In this virtual issue, we feature his Nobel Lecture as well as a selection of his papers published in Angewandte Chemie.