Formal Vinyl CH Activation and Allylic Oxidation by Olefin Metathesis
Arnab K. Chatterjee
Division of Chemistry and Chemical Engineering Arnold and Mabel Beckman Laboratories for Chemical Synthesis California Institute of Technology Mail Code 164-30, Pasadena, CA 91125 (USA) Fax: (+1) 626-564-9297
Search for more papers by this authorRobert H. Grubbs Prof. Dr.
Division of Chemistry and Chemical Engineering Arnold and Mabel Beckman Laboratories for Chemical Synthesis California Institute of Technology Mail Code 164-30, Pasadena, CA 91125 (USA) Fax: (+1) 626-564-9297
Search for more papers by this authorArnab K. Chatterjee
Division of Chemistry and Chemical Engineering Arnold and Mabel Beckman Laboratories for Chemical Synthesis California Institute of Technology Mail Code 164-30, Pasadena, CA 91125 (USA) Fax: (+1) 626-564-9297
Search for more papers by this authorRobert H. Grubbs Prof. Dr.
Division of Chemistry and Chemical Engineering Arnold and Mabel Beckman Laboratories for Chemical Synthesis California Institute of Technology Mail Code 164-30, Pasadena, CA 91125 (USA) Fax: (+1) 626-564-9297
Search for more papers by this authorGraphical Abstract
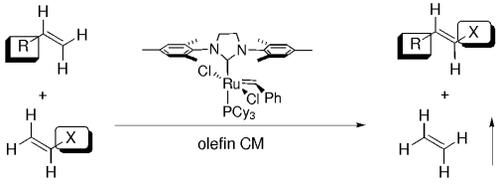
Stereoselective and chemoselective olefin cross metathesis can be viewed as a highly selective and efficient set of reactions that provide the same products as would selective CH activation and allylic oxidation (see scheme for an example). More active catalyst systems will provide an efficient process to functionalized products from readily available olefins. Cy=cyclohexyl.
References
- 1
- 1aA. Fürstner, Angew. Chem. 2000, 112, 3013;
10.1002/1521-3757(20000818)112:16<3013::AID-ANGE3013>3.0.CO;2-T Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3140;10.1002/1521-3773(20000901)39:17<3140::AID-ANIE3140>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 1bT. M. Trnka, R. H. Grubbs, Acc. Chem. Res. 2001, 34, 18;
- 1cS. Kotha, N. Sreenivasachary, Indian J. Chem. Sect. B 2001, 40, 763.
- 2Ill-defined catalyst systems were originally disclosed in CM by the following:
- 2aD. S. Banasiak, J. Mol. Catal. 1985, 28, 107;
- 2bS. Warwel, W. Winkelmuller, J. Mol. Catal. 1985, 28, 247.
- 3For a set of leading CM references with 1 and 2, see:
- 3aW. E. Crowe, D. R. Goldberg, J. Am. Chem. Soc. 1995, 117, 5162;
- 3bO. Brummer, A. Ruckert, S. Blechert, Chem. Eur. J. 1997, 3, 441;
- 3cH. E. Blackwell, D. J. O'Leary, A. K. Chatterjee, R. A. Washenfelder, D. A. Bussmann, R. H. Grubbs, J. Am. Chem. Soc. 2000, 122, 58.
- 4
- 4aM. Scholl, S. Ding, C. W. Lee, R. H. Grubbs, Org. Lett. 1999, 1, 953;
- 4bM. S. Sanford, J. A. Love, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 6543.
- 5
- 5aA. K. Chatterjee, J. P. Morgan, M. Scholl, R. H. Grubbs, J. Am. Chem. Soc. 2000, 122, 3783;
- 5bT. Choi, A. K. Chatterjee, R. H. Grubbs, Angew. Chem. 2001, 113, 1317;
Angew. Chem. Int. Ed. 2001, 40, 1277;
10.1002/1521-3773(20010401)40:7<1277::AID-ANIE1277>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 5b2A. K. Chatterjee, T. Choi, R. H. Grubbs, Synlett 2001, 1034;
- 5cT. Choi, C. W. Lee, A. K. Chatterjee, R. H. Grubbs, J. Am. Chem. Soc. 2001, 123, 10 417.
- 6
- 6aT. Ishiyama, J. Takagi, K. Ishida, N. Miyaura, N. R. Anastasi, J. F. Hartwig, J. Am. Chem. Soc. 2002, 124, 390;
- 6bJ.-Y. Cho, M. K. Tse, D. Holmes, R. E. Maleczka, M. R. Smith III, Science 2002, 295, 305.
- 7For CM of siloxanes in the presence of 2, see:
- 7aC. Pietraszuk, H. Fischer, M. Kujawa, B. Marciniec, Tetrahedron Lett. 2001, 42, 1175.
- 8
- 8aL.-B. Han, M. Tanaka, J. Am. Chem. Soc. 1996, 118, 1571.
- 9For examples, see:
- 9aN. Miyaura, A. Suzuki, Chem. Rev. 1995, 95, 2457;
- 9bT. Moriya, N. Miyaura, A. Suzuki, Chem. Lett. 1993, 1429;
- 9cS. Pereira, M. Srebnik, Organometallics 1995, 14, 3127.
- 10
- 10aA. K. Chatterjee, R. H. Grubbs, Org. Lett. 1999, 1, 1751;
- 10bA. K. Chatterjee, D. P. Sanders, R. H. Grubbs, Org. Lett. 2002, 4, 1939;
- 10cS. J. Spessard, B. M. Stoltz, Org. Lett. 2002, 4, 1943.



41:17<>1.0.co;2-c.cover.gif)
