Highly Active Chiral Ruthenium Catalysts for Asymmetric Cross- and Ring-Opening Cross-Metathesis†
Jacob M. Berlin
Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, Mail Code 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorSteven D. Goldberg Dr.
Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, Mail Code 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorRobert H. Grubbs Prof.
Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, Mail Code 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorJacob M. Berlin
Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, Mail Code 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorSteven D. Goldberg Dr.
Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, Mail Code 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorRobert H. Grubbs Prof.
Arnold and Mabel Beckman Laboratories of Chemical Synthesis, California Institute of Technology, Division of Chemistry and Chemical Engineering, Mail Code 164-30, Pasadena, CA 91125, USA, Fax: (+1) 626-564-9297
Search for more papers by this authorWe gratefully acknowledge Prof. F. D. Toste for early experimental work.
Graphical Abstract
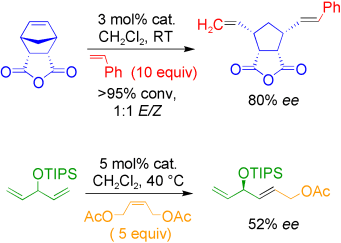
Metathesis takes sides: The scope of asymmetric metathesis has been expanded with the use of chiral ruthenium catalysts for asymmetric ring-opening cross-metathesis and for the first example of an asymmetric cross-metathesis (see scheme, TIPS=triisopropylsilyl). Information about the mechanism of asymmetric ring-opening cross-metathesis should allow the development of more selective catalysts.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2006/z602469_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1For recent reviews on olefin metathesis, see:
- 1aR. H. Grubbs, Handbook of Metathesis, Wiley-VCH, Weinheim, 2003;
10.1002/9783527619481 Google Scholar
- 1bA. Fürstner, Angew. Chem. 2000, 112, 3140–3172;
10.1002/1521-3757(20000901)112:17<3140::AID-ANGE3140>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2000, 39, 3012–3043;10.1002/1521-3773(20000901)39:17<3012::AID-ANIE3012>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 1cR. H. Grubbs, S. Chang, Tetrahedron 1998, 54, 4413–4450;
- 1dK. J. Ivin, J. Mol. Catal. A 1998, 133, 1–16.
- 2For reviews of molybdenum-catalyzed enantioselective metathesis, see:
- 2aR. R. Schrock, A. H. Hoveyda, Angew. Chem. 2003, 115, 4740–4782;
10.1002/ange.200300576 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 4592–4633; ;
- 2bA. H. Hoveyda, R. R. Schrock, Chem. Eur. J. 2001, 7, 945–950.
10.1002/1521-3765(20010302)7:5<945::AID-CHEM945>3.0.CO;2-3 CAS PubMed Web of Science® Google Scholar
- 3aS. J. Dolman, K. C. Hultzsch, F. Pezet, X. Teng, A. H. Hoveyda, R. R. Schrock, J. Am. Chem. Soc. 2004, 126, 10945–10953;
- 3bJ. A. Jernelius, R. R. Schrock, A. H. Hoveyda, Tetrahedron 2004, 60, 7345–7351;
- 3cS. J. Dolman, R. R. Schrock, A. H. Hoveyda, Org. Lett. 2003, 5, 4899–4902;
- 3dW. C. P. Tsang, J. A. Jernelius, G. A. Cortez, G. S. Weatherhead, R. R. Schrock, A. H. Hoveyda, J. Am. Chem. Soc. 2003, 125, 2591–2596.
- 4For a preparation of chiral molybdenum catalysts in situ, see: S. L. Aeilts, D. R. Cefalo, P. J. Bonitatebus, J. H. Houser, A. H. Hoveyda, R. R. Schrock, Angew. Chem. 2001, 113, 1500–1504;
10.1002/1521-3757(20010417)113:8<1500::AID-ANGE1500>3.0.CO;2-I Google ScholarAngew. Chem. Int. Ed. 2001, 40, 1452–1456.10.1002/1521-3773(20010417)40:8<1452::AID-ANIE1452>3.0.CO;2-G CAS PubMed Web of Science® Google Scholar
- 5Catalysts 4a,b have not been previously reported, but were prepared analogously to 2a,b, 3a,b, 5a,b, and 6a,b. For full experimental details, see the Supporting Information.
- 6
- 6aT. W. Funk, J. M. Berlin, R. H. Grubbs, J. Am. Chem. Soc. 2006, 128, 1840–1846;
- 6bT. J. Seiders, D. W. Ward, R. H. Grubbs, Org. Lett. 2001, 3, 3225–3228.
- 7On comparison with the results from references [6a] and [8c].
- 8
- 8aJ. J. Van Veldhuizen, J. E. Campbell, R. E. Giudici, A. H. Hoveyda, J. Am. Chem. Soc. 2005, 127, 6877–6882;
- 8bD. G. Gillingham, O. Kataoka, S. B. Garber, A. H. Hoveyda, J. Am. Chem. Soc. 2004, 126, 12288–12290;
- 8cJ. J. Van Veldhuizen, D. G. Gillingham, S. B. Garber, O. Kataoka, A. H. Hoveyda, J. Am. Chem. Soc. 2003, 125, 12502–12508;
- 8dJ. J. Van Veldhuizen, S. B. Garber, J. S. Kingsbury, A. H. Hoveyda, J. Am. Chem. Soc. 2002, 124, 4954–4955.
- 9For evidence for cis coordination, see:
- 9aT. M. Trnka, M. W. Day, R. H. Grubbs, Organometallics 2001, 20, 3845–3847; for evidence for trans coordination, see:
- 9bJ. A. Tallarico, P. J. Bonitatebus, Jr.,M. L. Snapper, J. Am. Chem. Soc. 1997, 119, 7157–7158;
- 9cP. E. Romero, W. E. Piers, J. Am. Chem. Soc. 2005, 127, 5032–5033.
- 10
- 10aC. Costabile, L. Cavallo, J. Am. Chem. Soc. 2004, 126, 9592–9600;
- 10bC. Adlhart, P. Chen, J. Am. Chem. Soc. 2004, 126, 3496–3510;
- 10cS. Fomine, S. M. Vargas, M. Tlenkopatchev, Organometallics 2003, 22, 93–99;
- 10dL. Cavallo, J. Am. Chem. Soc. 2002, 124, 8965–8973;
- 10eS. F. Vyboishchikov, M. Bühl, W. Thiel, Chem. Eur. J. 2002, 8, 3962–3975.
10.1002/1521-3765(20020902)8:17<3962::AID-CHEM3962>3.0.CO;2-X CAS PubMed Web of Science® Google Scholar
- 11The absolute stereochemistry of 9 has been determined by analysis of the X-ray crystal structure of an imide derivative (see the Supporting Information). From the comparison of HPLC traces run under identical conditions,our studies indicate that the incorrect absolute stereoisomer was reported in reference [8d].
- 12For example, see: H. C. Brown, N. R. Ayyangar, G. Zweifel, J. Am. Chem. Soc. 1964, 86, 397–403.
- 13A. K. Chatterjee, T.-L. Choi, D. P. Sanders, R. H. Grubbs, J. Am. Chem. Soc. 2003, 125, 11360–11370, and references therein.