Electrical Contacting of Glucose Oxidase in a Redox-Active Rotaxane Configuration†
Eugenii Katz Dr.
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel, Fax: (+972) 2-652-7715
Search for more papers by this authorLaila Sheeney-Haj-Ichia
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel, Fax: (+972) 2-652-7715
Search for more papers by this authorItamar Willner Prof.
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel, Fax: (+972) 2-652-7715
Search for more papers by this authorEugenii Katz Dr.
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel, Fax: (+972) 2-652-7715
Search for more papers by this authorLaila Sheeney-Haj-Ichia
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel, Fax: (+972) 2-652-7715
Search for more papers by this authorItamar Willner Prof.
Institute of Chemistry, The Hebrew University of Jerusalem, Jerusalem 91904, Israel, Fax: (+972) 2-652-7715
Search for more papers by this authorThis research (No. 101/00) is supported by the Israel Science Foundation.
Graphical Abstract
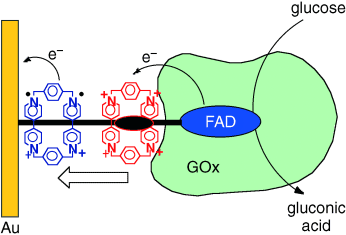
Shuttle of electrons: A redox-active bisbipyridinium cyclophane stoppered by the enzyme glucose oxidase (GOx) in a rotaxane configuration on a molecular wire linked to a gold electrode (see picture) leads to the effective electrical contacting of the biocatalyst that oxidizes glucose at −0.4 V (versus saturated calomel electrode).
References
- 1
- 1aE. Katz, A. N. Shipway, I. Willner in Encyclopedia of Electrochemistry, Vol. 9 (Eds.: ), Wiley-VCH, Weinheim, 2002, chap. 17, pp. 559– 626;
- 1bI. Willner, E. Katz, Angew. Chem. 2000, 112, 1230–1269;
Angew. Chem. Int. Ed. 2000, 39, 1180–1218;
10.1002/(SICI)1521-3773(20000403)39:7<1180::AID-ANIE1180>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 1cA. Heller, Acc. Chem. Res. 1990, 23, 128–134;
- 1dF. A. Armstrong, G. S. Wilson, Electrochim. Acta 2000, 45, 2623–2645.
- 2
- 2aE. Katz, A. N. Shipway, I. Willner in Handbook of Fuel Cells—Fundamentals, Technology, Applications, Vol. 1, Part 4 (Eds.: ), Wiley, New York, 2003, chap. 21, pp. 355– 381;
- 2bE. Katz, I. Willner, A. B. Kotlyar, J. Electroanal. Chem. 1999, 479, 64–68;
- 2cE. Katz, I. Willner, J. Am. Chem. Soc. 2003, 125, 6803–6813;
- 2dN. Mano, F. Mao, A. Heller, J. Am. Chem. Soc. 2003, 125, 6588–6594.
- 3
- 3aY. Degani, A. Heller, J. Phys. Chem. 1987, 91, 1285–1289;
- 3bW. Schuhmann, T. J. Ohara, H.-L. Schmidt, A. Heller, J. Am. Chem. Soc. 1991, 113, 1394–1397;
- 3cI. Willner, A. Riklin, B. Shoham, D. Rivenzon, E. Katz, Adv. Mater. 1993, 5, 912–915.
- 4
- 4aA. Heller, J. Phys. Chem. 1992, 96, 3579–3587;
- 4bH. Bu, S. R. Mikkelsen, A. M. English, Anal. Chem. 1995, 67, 4071–4076.
- 5
- 5aI. Willner, V. Heleg-Shabtai, R. Blonder, E. Katz, G. Tao, A. F. Bückmann, A. Heller, J. Am. Chem. Soc. 1996, 118, 10 321–10 322;
- 5bE. Katz, A. Riklin, V. Heleg-Shabtai, I. Willner, A. F. Bückmann, Anal. Chim. Acta 1999, 385, 45–58.
- 6Y. Xiao, F. Patolsky, E. Katz, J. F. Hainfeld, I. Willner, Science 2003, 299, 1877–1881.
- 7F. Patolsky, Y. Weizmann, I. Willner, Angew. Chem. 2004, 116, 2165–2169;
10.1002/ange.200353275 Google ScholarAngew. Chem. Int. Ed. 2004, 43, 2113–2117.
- 8
- 8aR. A. Bissell, E. Cordova, A. E. Kaifer, J. F. Stoddart, Nature 1994, 369, 133–137;
- 8bM. C. T. Fyfe, J. F. Stoddart, Acc. Chem. Res. 1997, 30, 393–401;
- 8cH. R. Tseng, S. A. Vignon, J. F. Stoddart, Angew. Chem. 2003, 115, 1529–1533;
10.1002/ange.200250453 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 1491–1495;
- 8dS. J. Rowan, J. F. Stoddart, Polym. Adv. Technol. 2002, 13, 777–787;
- 8eS. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders, J. F. Stoddart, Angew. Chem. 2002, 114, 1528–1531;
10.1002/1521-3757(20020503)114:9<1528::AID-ANGE11111528>3.0.CO;2-Z Google ScholarAngew. Chem. Int. Ed. 2002, 41, 898–952.10.1002/1521-3773(20020315)41:6<898::AID-ANIE898>3.0.CO;2-E CAS PubMed Web of Science® Google Scholar
- 9
- 9aM. Lahav, A. N. Shipway, I. Willner, J. Chem. Soc. Perkin Trans. 2 1999, 9, 1925–1931;
- 9bA. B. Kharitonov, A. N. Shipway, I. Willner, Anal. Chem. 1999, 71, 5441–5443;
- 9cI. Willner, V. Pardo-Yissar, E. Katz, K. T. Ranjit, J. Electroanal. Chem. 2001, 497, 172–177;
- 9dC. P. Collier, E. W. Wong, M. Belohradsky, F. M. Raymo, J. F. Stoddart, P. J. Kuekes, R. S. Williams, J. R. Heath, Science 1999, 285, 391–394;
- 9eY. Luo, C. P. Collier, J. O. Jeppesen, K. A. Nielsen, E. Delonno, G. Ho, J. Perkins, H. R. Tseng, T. Yamamoto, J. F. Stoddart, J. R. Heath, ChemPhysChem 2002, 3, 519–525.
10.1002/1439-7641(20020617)3:6<519::AID-CPHC519>3.0.CO;2-2 CAS PubMed Web of Science® Google Scholar
- 10
- 10aL. Sheeney-Haj-Ichia, I. Willner, J. Phys. Chem. B 2002, 106, 13 094–13 097;
- 10bM. Asakawa, P. R. Ashton, V. Balzani, C. L. Brown, A. Credi, O. A. Matthews, S. P. Newton, F. M. Raymo, A. N. Shipway, N. Spencer, A. Quick, J. F. Stoddart, A. J. P. White, D. J. Williams, Chem. Eur. J. 1999, 5, 860–875;
- 10cP. R. Ashton, V. Balzani, O. Kocian, L. Prodi, N. Spencer, J. F. Stoddart, J. Am. Chem. Soc. 1998, 120, 11 190–11 191.
- 11
- 11aP. J. Elving, J. E. O'Reilly, C. O. Schmakel in Methods of Biochemical Analysis, Vol. 21 (Ed.: ), Interscience, New York, 1973;
- 11bG. Dryhurst, Electrochemistry of Biological Molecules, Academic Press, New York, 1977.
- 12
- 12aE. Katz, D. D. Schlereth, H.-L. Schmidt, J. Electroanal. Chem. 1994, 367, 59–70;
- 12bE. Katz, D. D. Schlereth, H.-L. Schmidt, A. J. J. Olsthoorn, J. Electroanal. Chem. 1994, 368, 165–171.
- 13
- 13aF. C. Anson, Anal. Chem. 1966, 38, 54–57;
- 13bA. B. Steel, T. M. Herne, M. J. Tarlov, Anal. Chem. 1998, 70, 4670–4677.
- 14D. A. Buttry, M. D. Ward, Chem. Rev. 1992, 92, 1355–1379.
- 15A. R. Bernardo, J. F. Stoddart, A. E. Kaifer, J. Am. Chem. Soc. 1992, 114, 10 624–10 631.
- 16The surface coverages cannot be directly derived from the DPVs; they were derived from a cyclic voltammogram recorded at pH 9.5 at which the FAD potential is negatively shifted (δE°/δpH≈30 mV pH−1 at pH>7) relative to the pH-independent potential of 2, to provide separate waves for both redox components in the cyclic voltammogram.
- 17M. Zayats, E. Katz, I. Willner, J. Am. Chem. Soc. 2002, 124, 14 724–14 735.
- 18The GOx-reconstituted electrode, which lacks the interlocked rotaxane-mediator and thus does not show bioelectrocatalytic oxidation of glucose, was activated in the presence of the diffusional electron-transfer mediator, ferrocene monocarboxylic acid (0.5 mM). Therefore, the reconstituted GOx exists on the surface in the biocatalytically active state.
- 19
- 19aR. J. Forster, L. R. Faulkner, Anal. Chem. 1995, 67, 1232–1239;
- 19bR. J. Forster, Langmuir 1995, 11, 2247–2255;
- 19cR. J. Forster, Anal. Chem. 1996, 68, 3143–3150;
- 19dR. J. Forster, Analyst 1996, 121, 733–741.
- 20E. Katz, I. Willner, Langmuir 1997, 13, 3364–3373.
- 21V. Pardo-Yissar, E. Katz, I. Willner, A. B. Kotlyar, C. Sanders, H. Lill, Faraday Discuss. 2000, 116, 119–134.
- 22D. L. Morris, R. T. Buckler, Methods Enzymol. 1983, 92, 413–417.
- 23A. F. Bückmann, V. Wray, A. Stocker in Methods in Enzymology: Vitamins and Coenzymes, Vol. 280, Part 1 (Ed.: ), Academic Press, Orlando, 1997, p. 360.
- 24M. Asakawa, W. Dehaen, G. Labbe, S. Menzer, J. Nouwen, F. M. Raymo, J. F. Stoddart, D. J. Williams, Org. Chem. 1996, 61, 9591–9595.
- 25A. J. Bard, L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, 1980.