Kinetic Resolution of Amines: A Highly Enantioselective and Chemoselective Acetylating Agent with a Unique Solvent-Induced Reversal of Stereoselectivity†
Stellios Arseniyadis Dr.
Laboratoire de Synthèse Bioorganique, UMR 7514 du CNRS, Faculté de Pharmacie, 74 route du Rhin, 67400 Illkirch, France, Fax: (+33) 3-9024-4306
Search for more papers by this authorAlain Valleix
CEA Saclay, Service des molecules marquées 91191 Gif sur Yvette, France
Search for more papers by this authorAlain Wagner Dr.
Laboratoire de Synthèse Bioorganique, UMR 7514 du CNRS, Faculté de Pharmacie, 74 route du Rhin, 67400 Illkirch, France, Fax: (+33) 3-9024-4306
Search for more papers by this authorCharles Mioskowski Dr.
Laboratoire de Synthèse Bioorganique, UMR 7514 du CNRS, Faculté de Pharmacie, 74 route du Rhin, 67400 Illkirch, France, Fax: (+33) 3-9024-4306
CEA Saclay, Service des molecules marquées 91191 Gif sur Yvette, France
Search for more papers by this authorStellios Arseniyadis Dr.
Laboratoire de Synthèse Bioorganique, UMR 7514 du CNRS, Faculté de Pharmacie, 74 route du Rhin, 67400 Illkirch, France, Fax: (+33) 3-9024-4306
Search for more papers by this authorAlain Valleix
CEA Saclay, Service des molecules marquées 91191 Gif sur Yvette, France
Search for more papers by this authorAlain Wagner Dr.
Laboratoire de Synthèse Bioorganique, UMR 7514 du CNRS, Faculté de Pharmacie, 74 route du Rhin, 67400 Illkirch, France, Fax: (+33) 3-9024-4306
Search for more papers by this authorCharles Mioskowski Dr.
Laboratoire de Synthèse Bioorganique, UMR 7514 du CNRS, Faculté de Pharmacie, 74 route du Rhin, 67400 Illkirch, France, Fax: (+33) 3-9024-4306
CEA Saclay, Service des molecules marquées 91191 Gif sur Yvette, France
Search for more papers by this authorWe thank Rhodia for financial support to S.A., as well as Dr. A. De Cian and Dr. N. Gruber for their helpful collaborations in the collection of the X-ray crystallographic data.
Graphical Abstract
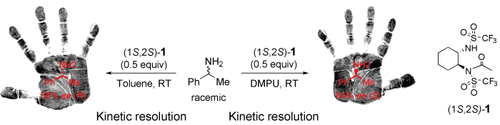
Solvents lend a hand: Changing the polarity of the reaction solvent from 1,3-dimethyltetrahydropyrimidin-2-one (DMPU) to toluene reverses the stereoselectivity observed in the acetylation of amines with (1S,2S)-1 (see scheme). Optimizing the reaction conditions led to an unprecedented 90 % ee (S) in DMPU at −20 °C with a 33 % conversion.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53956_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1U. T. Bornscheuer, R. J. Kazlauskas, Hydrolases in Organic Synthesis. Regio- and Stereoselective Biotransformations, Wiley-VCH, Weinheim, 1999.
- 2
- 2aA. C. Spivey, A. Maddaford, A. Redgrave, Org. Prep. Proced. Int. 2000, 32, 331;
- 2bS. M. Roberts, J. Chem. Soc. Perkin Trans. 1 1999, 1.
- 3G. Zanoni, F. Castronovo, M. Franzini, G. Vidari, E. Giannini, Chem. Soc. Rev. 2003, 32, 115.
- 4D. A. Evans, J. C. Anderson, M. K. Taylor, Tetrahedron Lett. 1993, 34, 5563.
- 5E. Vedejs, X. Chen, J. Am. Chem. Soc. 1996, 118, 1809.
- 6Selectivity factor s=(rate of fast-reacting enantiomer)/(rate of slow-reacting enantiomer); for a review of kinetic resolution, see: H. B. Kagan, J. C. Fiaud, Top. Stereochem. 1998, 18, 249.
10.1002/9780470147276.ch4 Google Scholar
- 7E. Vedejs, O. Daugulis, S. T. Diver, J. Org. Chem. 1996, 61, 430.
- 8T. Oriyama, Y. Hori, K. Imai, R. Sasaki, Tetrahedron Lett. 1996, 37, 8543.
- 9T. Kawabata, M. Nagato, K. Takasu, K. Fuji, J. Am. Chem. Soc. 1997, 119, 3169.
- 10
- 10aS. J. Miller, G. T. Copeland, N. Papaioannou, T. E. Horstmann, E. M. Ruel, J. Am. Chem. Soc. 1998, 120, 1629;
- 10bE. R. Jarvo, G. T. Copeland, N. Papaioannou, P. J. Bonitatebus, S. J. Miller, J. Am. Chem. Soc. 1999, 121, 11 638.
- 11
- 11aJ. C. Ruble, G. C. Fu, J. Org. Chem. 1996, 61, 7230;
- 11bJ. C. Ruble, H. A. Latham, G. C. Fu, J. Am. Chem. Soc. 1997, 119, 1492;
- 11cB. Tao, J. C. Ruble, D. A. Hoic, G. C. Fu, J. Am. Chem. Soc. 1999, 121, 5091;
- 11dS. Bellemin-Laponnaz, J. Tweddell, J. C. Ruble, F. M. Breitling, G. C. Fu, Chem. Commun. 2000, 1009;
- 11eJ. C. Ruble, J. Tweddell, G. C. Fu, J. Org. Chem. 1998, 63, 2794;
- 11fG. C. Fu, Acc. Chem. Res. 2000, 33, 412.
- 12
- 12aA. C. Spivey, T. Fekner, S. E. Spey, J. Org. Chem. 2000, 65, 3154;
- 12bA. C. Spivey, A. Maddaford, T. Fekner, A. J. Redgrave, C. S. Frampton, J. Chem. Soc. Perkin Trans. 1 2000, 3460;
- 12cA. C. Spivey, F. Zhu, M. B. Mitchell, S. G. Davey, R. L. Jarvest, J. Org. Chem. 2003, 68, 7379.
- 13Besides displaying various biological activities, enantiopure amines are widely used as reagents in asymmetric synthesis, for example, see: E. Juaristi, J. L. Leon-Romo, A. Reyes, J. Escalante, Tetrahedron: Asymmetry 1999, 10, 2441.
- 14K. Kondo, T. Kurosaki, Y. Murakami, Synlett 1998, 725.
- 15The kinetic resolution of (±)-1-naphthylethylamine was obtained with up to 48 % ee using 0.25 equivalents of chiral reagent at room temperature. This ee value corresponds to a selectivity factor (s) of 3 at 25 % conversion.
- 16A. G. Al-Sehemi, R. S. Atkinson, J. Fawcett, D. R. Russell, Tetrahedron Lett. 2000, 41, 2239.
- 17Y. Ie, G. C. Fu, Chem. Commun. 2000, 119.
- 18Under these reaction conditions, Fu and co-workers achieved the kinetic resolution of (±)-1 phenylethylamine with 87 % ee.
- 19S. Arai, S. Bellemin-Laponnaz, G. C. Fu, Angew. Chem. 2001, 113, 667;
10.1002/1521-3757(20010216)113:4<667::AID-ANGE6674>3.0.CO;2-H Google ScholarAngew. Chem. Int. Ed. 2001, 40, 234.10.1002/1521-3773(20010105)40:1<234::AID-ANIE234>3.0.CO;2-K CAS PubMed Web of Science® Google Scholar
- 20This ee value corresponds to a selectivity factor (s) of 13 at 35 % conversion.
- 21For a review on chiral 1,2-diamines, see: D. Lucet, T. Le Gall, C. Mioskowski, Angew. Chem. 1998, 110, 2724;
10.1002/(SICI)1521-3757(19981002)110:19<2724::AID-ANGE2724>3.0.CO;2-4 Google ScholarAngew. Chem. Int. Ed. 1998, 37, 2580.10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L CAS PubMed Web of Science® Google Scholar
- 22For a review on solvents and solvent effects, see: C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, 3rd ed., Wiley–VCH, Weinheim, Germany, 2002.
10.1002/3527601791 Google Scholar