Aromatic Donor–Acceptor Charge-Transfer and Metal-Ion-Complexation-Assisted Folding of a Synthetic Polymer†
Suhrit Ghosh
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India, Fax: (+80) 2360-0683
Search for more papers by this authorS. Ramakrishnan Prof.
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India, Fax: (+80) 2360-0683
Search for more papers by this authorSuhrit Ghosh
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India, Fax: (+80) 2360-0683
Search for more papers by this authorS. Ramakrishnan Prof.
Department of Inorganic and Physical Chemistry, Indian Institute of Science, Bangalore 560012, India, Fax: (+80) 2360-0683
Search for more papers by this authorWe thank the DST, New Delhi, and 3M India Ltd, for their financial support.
Graphical Abstract
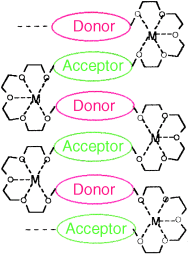
Designer folds: The interplay of three design elements (aromatic donor–acceptor charge-transfer complexation, solvophobic effect, and metal-ion complexation) guided the preparation of a folded structure (see picture) in a synthetic macromolecule. Evidence for this is provided by UV/Vis and 1H NMR spectroscopy.
Supporting Information
Supporting information for this article is available on the WWW under http://www.wiley-vch.de/contents/jc_2002/2004/z53860_s.pdf or from the author.
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1M. Muthukumar, C. K. Ober, E. L. Thomas, Science 1977, 277, 1225.
- 2S. H. Gellman, Acc. Chem. Res. 1998, 31, 173.
- 3
- 3aD. J. Hill, M. J. Mio, R. B. Prince, T. S. Huges, J. S. Moore, Chem. Rev. 2001, 101, 3893;
- 3bC. Schmuck, Angew. Chem. 2003, 115, 2552;
10.1002/ange.200201625 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 2448.
- 4T. Nakano, Y. Okamoto, Chem. Rev. 2001, 101, 4013.
- 5M. M. Green, J. W. Park, T. Sato, A. Teramoto, S, Lifson, R. L. B. Selinger, J. V. Selinger, Angew. Chem. 1999, 111, 3328;
10.1002/(SICI)1521-3757(19991102)111:21<3328::AID-ANGE3328>3.0.CO;2-Z Web of Science® Google ScholarAngew. Chem. Int. Ed. 1999, 38, 3138.10.1002/(SICI)1521-3773(19991102)38:21<3138::AID-ANIE3138>3.0.CO;2-C CAS PubMed Web of Science® Google Scholar
- 6K. Maeda, S. Okada, E. Yashima, Y. Okamoto, J. Polym. Sci., Part A: Polym. Chem. 2001, 39, 3180.
- 7J. J. L. M. Cornelissen, A. E. Rowan, R. J. M. Nolte, N. A. J. M. Sommerdijk, Chem. Rev. 2001, 101, 4039.
- 8J. C. Nelson, J. G. Saven, J. S. Moore, P. G. Wolynes, Science 1997, 277, 1793; a more recent report describes an elegant way to crosslink photochemically the helical conformation in high molecular weight poly(1,3-phenylene ethynylene)s:
R. S. Hecht, A. Khan, Angew. Chem. 2003, 115, 6203;
10.1002/ange.200352983 Google ScholarAngew. Chem. Int. Ed. 2003, 42, 6021.
- 9
- 9aR. S Lokey, B. L. Iverson, Nature 1995, 375, 303;
- 9bJ. Q. Nguyen, B. L. Iverson, J. Am. Chem. Soc. 1999, 121, 2639;
- 9cA. J. Zych, B. L. Iverson, J. Am. Chem. Soc. 2000, 122, 8898;
- 9dG. J. Gabriel, B. L. Iverson, J. Am. Chem. Soc. 2002, 124, 15 174.
- 10For well-defined oligomers containing perylene units linked by oligoethylene glycol units, see:
- 10aW. Wang, L-S. Li, G. Helms, H-H. Zhou, A. D. Q. Li, J. Am. Chem. Soc. 2003, 125, 1120;
- 10bA. D. Q. Li, W. Wang, L-Q. Wang, Chem. Eur. J. 2003, 9, 4594;
- 10cfor a postulated self-assembly in similar polymeric systems containing perylene bisimide units linked through flexible spacers, see:E. E. Neuteboom, S. C. J. Meskers, E. W. Meijer, R. A. J. Janssen, Macromol. Chem. Phys. 2004, 205, 217.
- 11Early comprehensive studies on podands clearly demonstrated the possibility of open-chain analogues of crown ethers to complex with metal ions and generate folded structures. Several workers exploited such metal-ion-induced folding to modulate photophysical behavior in small molecules. For comprehensive reviews, see:
- 11aF. Vogtle, E. Weber, Angew. Chem. 1979, 91, 813;
10.1002/ange.19790911007 Google ScholarAngew. Chem. Int. Ed. Engl. 1979, 18, 753;
- 11bH. G. Lohr, F. Vogtle, Acc. Chem. Res. 1985, 18, 65; for examples of photophysical studies, see:
- 11cH. G. Lohr, F. Vogtle, Chem. Ber. 1985, 118, 914;
- 11dA. Ajayaghosh, E. Arunkumar, J. Daub, Angew. Chem. 2002, 114, 1844;
10.1002/1521-3757(20020517)114:10<1844::AID-ANGE1844>3.0.CO;2-G Google ScholarAngew. Chem. Int. Ed. 2002, 41, 1766;10.1002/1521-3773(20020517)41:10<1766::AID-ANIE1766>3.0.CO;2-4 CAS PubMed Web of Science® Google Scholar
- 11eY. Suzuku, T. Morozumi, H. Nakamura, M. Shimomura, T. Hayashita, R. A. Bartsh, J. Phys. Chem. B 1998, 102, 7910;
- 11fB. Tummler, G. Maass, F. Vogtle, H. Sieger, U. Heimann, E. Weber, J. Am. Chem. Soc. 1979, 101, 2588.
- 12Experimental details regarding the synthesis and characterization of the monomer and the polymer are available in the Supporting Information.
- 13In contrast, the homopolyimide, which is devoid of the naphthalene unit, prepared from simple amine terminated oligoethylene glycol and pyromellitic dianhydride, is white in colour.
- 141,5-Dimethoxynaphthalene was used as the model donor and the pyromellitic diimide prepared from pyromellitic dianhydride with 2-[2-(2-methoxyethoxy)ethoxy]ethylene amine was used as the model acceptor.
- 15For examples of small molecule systems in which enhanced charge-transfer complexation results from linking of the donor and acceptor units, see:
- 15aN. C Yang, Y. Gaoni, J. Am. Chem. Soc. 1964, 86, 5022;
- 15bJ. W. Verhoeven, I. P. Drikx, T. J. Deboer, Tetrahedron Lett. 1966, 7, 439;
10.1016/S0040-4039(00)70048-7 Google Scholar
- 15cD. Kost, N. Peor, G. Sod-Moriah, Y. Sharabi, D. T. Durocher, M. Raban, J. Org. Chem. 2002, 67, 6938.
- 16
- 16aR. Forster in Organic Charge-Transfer Complex, Academic Press, London, 1969;
- 16bM. S. Cubberley, B. L. Iverson, J. Am. Chem. Soc. 2001, 123, 7560.
- 17A similar variation in the chemical shift of the acceptor protons, going through a maximum with increasing solvent polarity was noticed, which confirms the initial decrease prior to the increase in the apparent association constant. Detailed NMR spectral evolution as a function of solvent composition is included in the Supporting Information.
- 18Similar upfield shifts of the signals for the donor and acceptor protons upon charge-transfer complex formation was previously reported, and may be ascribed to the perturbation of the aromatic ring current causing a shielding effect:
- 18aY. Nakamura, S. Minami, K. Iizuka, J. Nishimura, Angew. Chem. 2003, 115, 3266; Angew. Chem. Int. Ed. 2003, 42, 3158; see also reference [9c].
- 19The chemical shift value of the acceptor proton in pure CDCl3 is 8.07 ppm as compared to 7.9 ppm in CDCl3/CH3CN (1:1 v/v). In contrast, there was no change in the chemical shifts in the case of a mixture of the model compounds at the same concentration.
- 20The program EQNMR version 2.10 was used to calculate the K values from the variation of the chemical shift of the acceptor proton. (Freeware developed by M. J. Hynes, Chemistry Department, University College, Galway, Ireland). It is evident that the association constant does not directly parallel the extent of change seen in either the charge-transfer absorbance or the chemical shift. It may be reasoned that the extent of change experienced by the donor and acceptor units (both in their NMR and UV/Vis spectra), owing to metal-ion complexation by the loop, would greatly depend on the final geometry of the donor–acceptor complex. Thus, formation of a strong complex with the metal ion need not necessarily bring the donor and acceptor units in the optimum geometry for most effective charge-transfer interaction. Further studies are currently being done on model conor–acceptor oligomers to gain better insight into these aspects.
- 21The maximum change is noticed for the methylene protons of the central region of the loop, which move downfield, whereas the methylene protons immediately adjacent to the chromophores move slightly upfield with K+ and Na+ and change little in the case of Li+. A similar variation was reported by Vogtle and co-workers in the case of podands (see reference [11a]). All the spectral variations are included in the Supporting Information.