Journal list menu
Export Citations
Download PDFs
Cover Picture
Cover Picture
- Page: 1941
- First Published: 01 August 2024

Dibenzo[b,f][1,5]diazocines are a class of eight-membered heterocycles with a rigid, saddle-shaped conformation that exhibit distinct inherent chirality. A straightforward catalytic enantioselective synthesis method for these saddle-shaped structures has been disclosed through the asymmetric dimerization of 2-acylbenzoisocyanates, enabled by the cooperative catalysis of chiral phosphoric acid and 2-acylanilines. More details are discussed in the article by Yang et al. on page 1953—1959.
Inside Cover Picture
Inside Cover Picture
- Page: 1942
- First Published: 01 August 2024

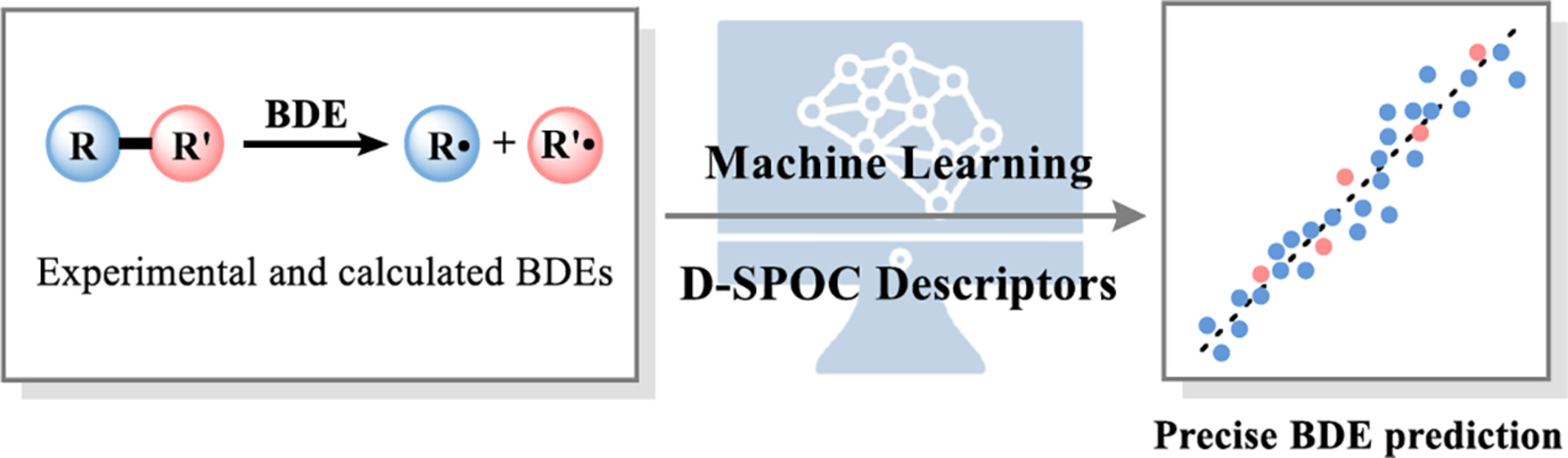
A machine learning-based comprehensive Bond Dissociation Energy (BDE) prediction model was established, which is useful for understanding the chemical properties and reactivities of molecules. Differential Structural and PhysicOChemical (D-SPOC) descriptors that reflected changes in molecules’ structural and physicochemical features in the process of bond homolysis were developed as input features, which enabled the precise prediction. More details are discussed in the article by Zhang et al. on page 1967—1974.
Contents
Breaking Report
Catalytic Asymmetric Synthesis of Inherently Chiral Saddle-Shaped Dibenzo[b,f][1,5]diazocines
- Pages: 1953-1959
- First Published: 20 April 2024
![Catalytic Asymmetric Synthesis of Inherently Chiral Saddle-Shaped Dibenzo[b,f][1,5]diazocines†](/cms/asset/5cc8b5d2-31f5-4f41-9cac-f6e275e26444/cjoc202400243-toc-0001-m.jpg)
Catalytic enantioselective synthesis of inherently chiral saddle-shaped dibenzo[b,f][1,5]diazocines was achieved through chiral phosphoric acid catalyzed asymmetric dimerization of 2-acylbenzoisocyanates. Remarkably, the incorporation of corresponding 2-acylaniline as a co-catalyst significantly enhanced the reaction efficiency, providing products with excellent enantiopurity through a straightforward phase separation process.
Concise Report
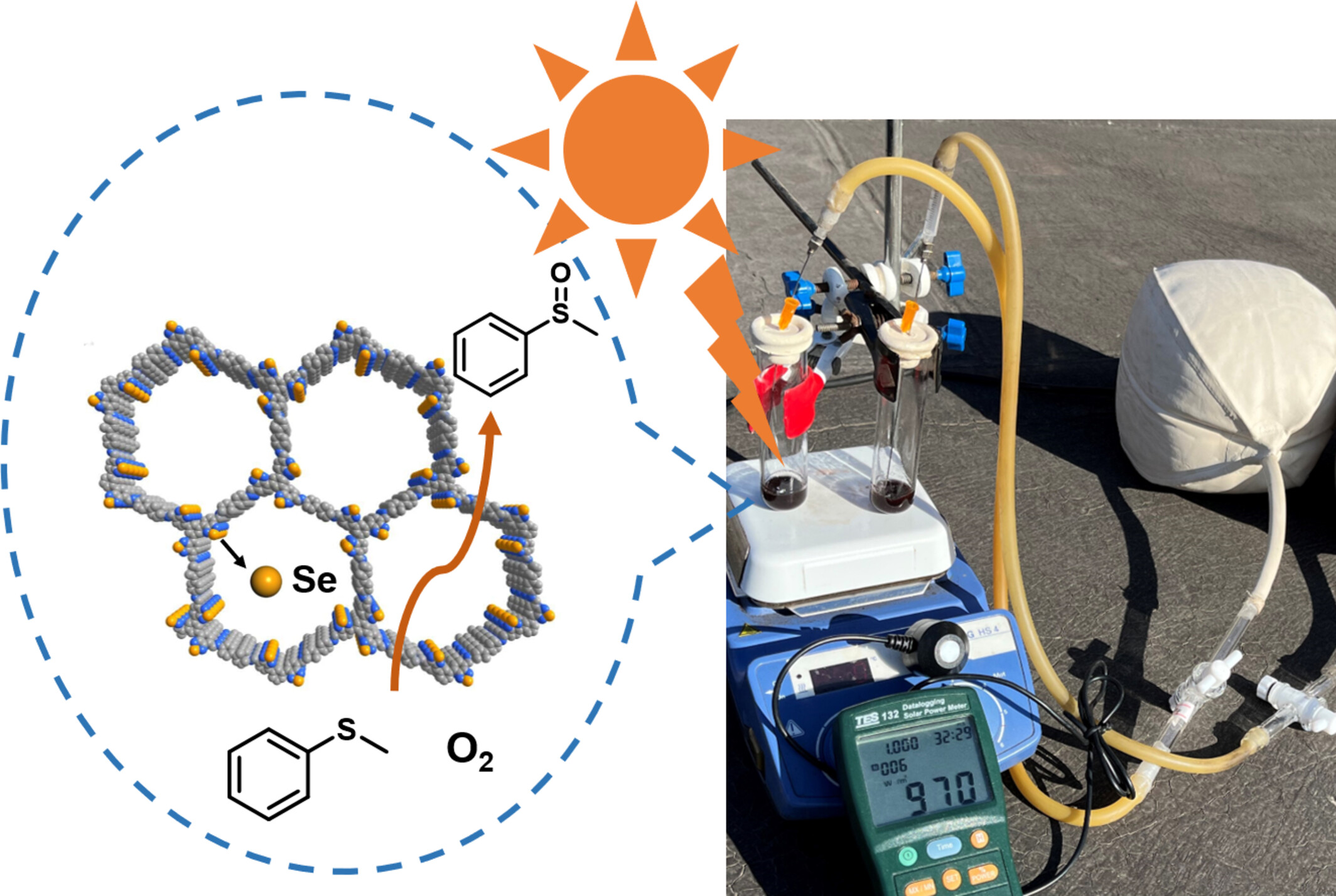
A Selenium Atom Involved Covalent Organic Framework for Window Ledge Photocatalytic Oxidation of Sulfides
- Pages: 1960-1966
- First Published: 20 April 2024
Prediction of Bond Dissociation Energy for Organic Molecules Based on a Machine-Learning Approach
- Pages: 1967-1974
- First Published: 20 April 2024
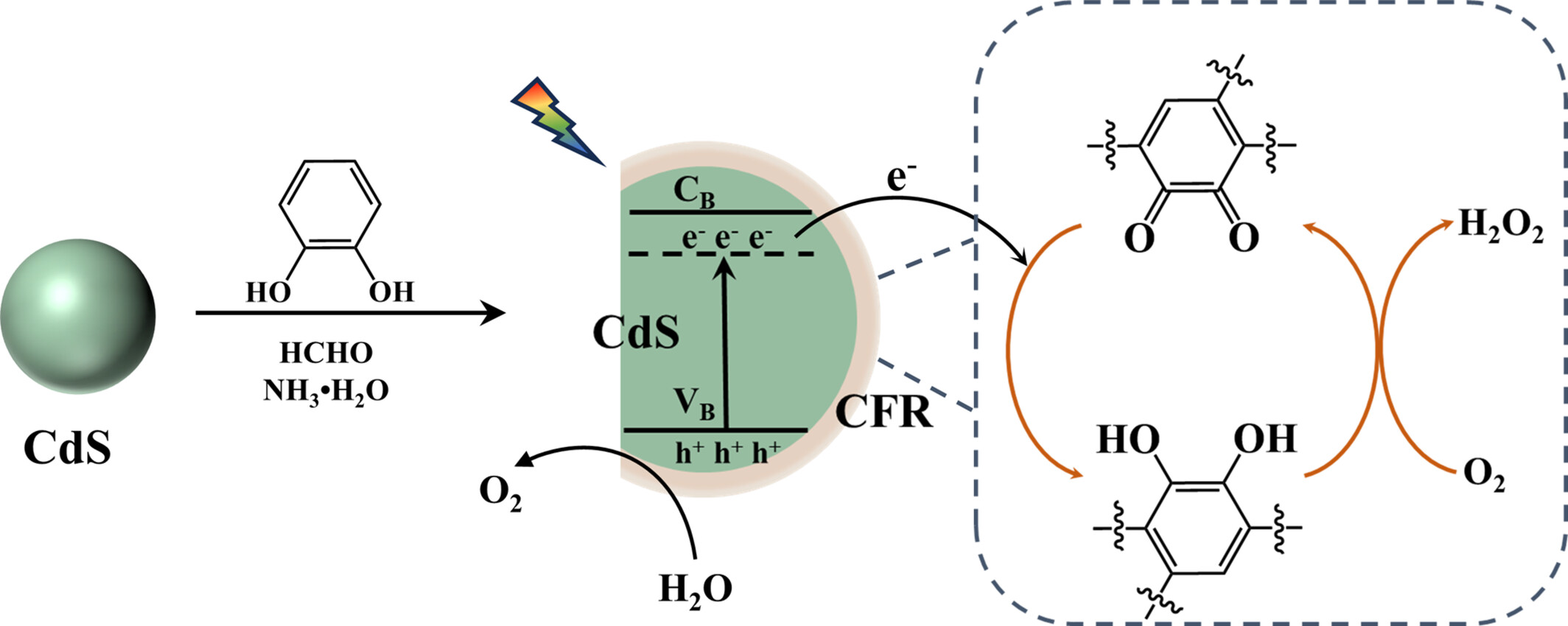
Catechol-Formaldehyde Resin Coated CdS Core-Shell Composite as Robust Photocatalyst for Long-Term Sustainable Artificial Photosynthesis of H2O2
- Pages: 1975-1985
- First Published: 30 April 2024
Rhodium-Catalyzed Regioselective C—O and C—C Bonds Formation of 3-Oxopent-4-enenitriles with Alkynes for the Synthesis of Polysubstituted 2H-Pyrans
- Pages: 1986-1992
- First Published: 25 April 2024
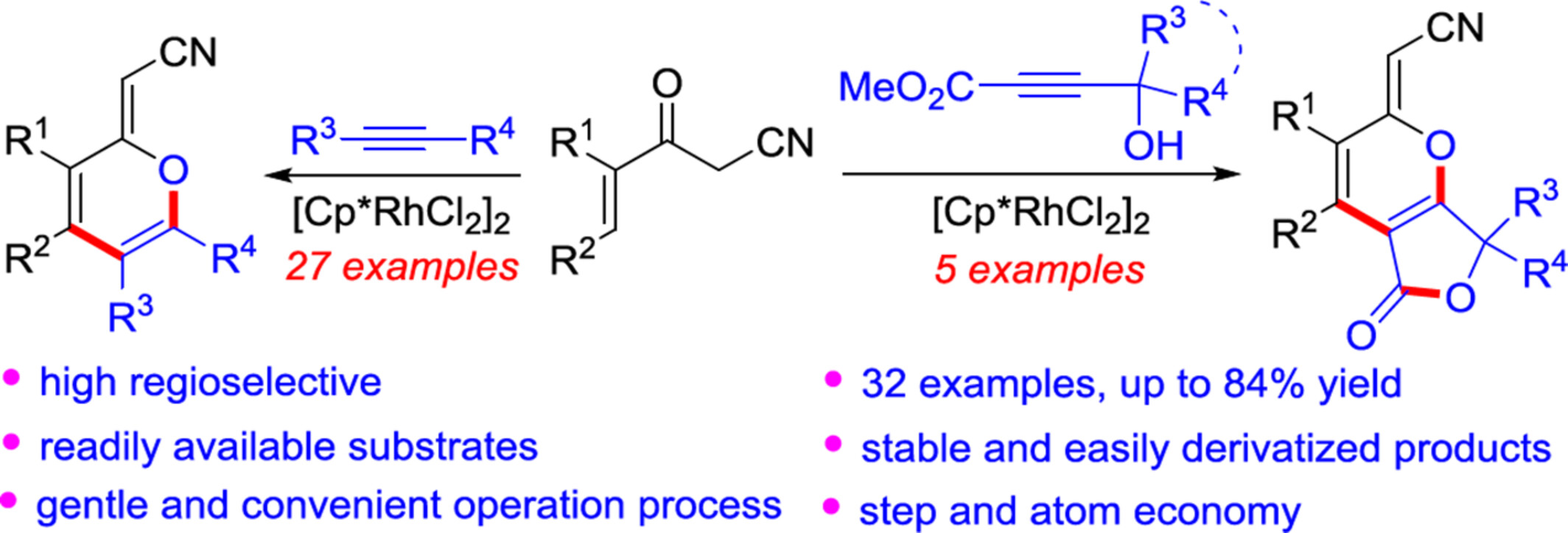
A rhodium-catalyzed regioselective C—O and C—C bonds formation of 3-oxopent-4-enenitriles with alkynes has been proposed for the synthesis of polysubstituted 2H-pyrans. Different from the traditional “1-oxatrienes pathway”, this method for the synthesis of useful 2H-pyrans possesses certain highlights in terms of readily available substrates, stable and easily derivatized products, gentle and convenient operation process, and step and atom economy.
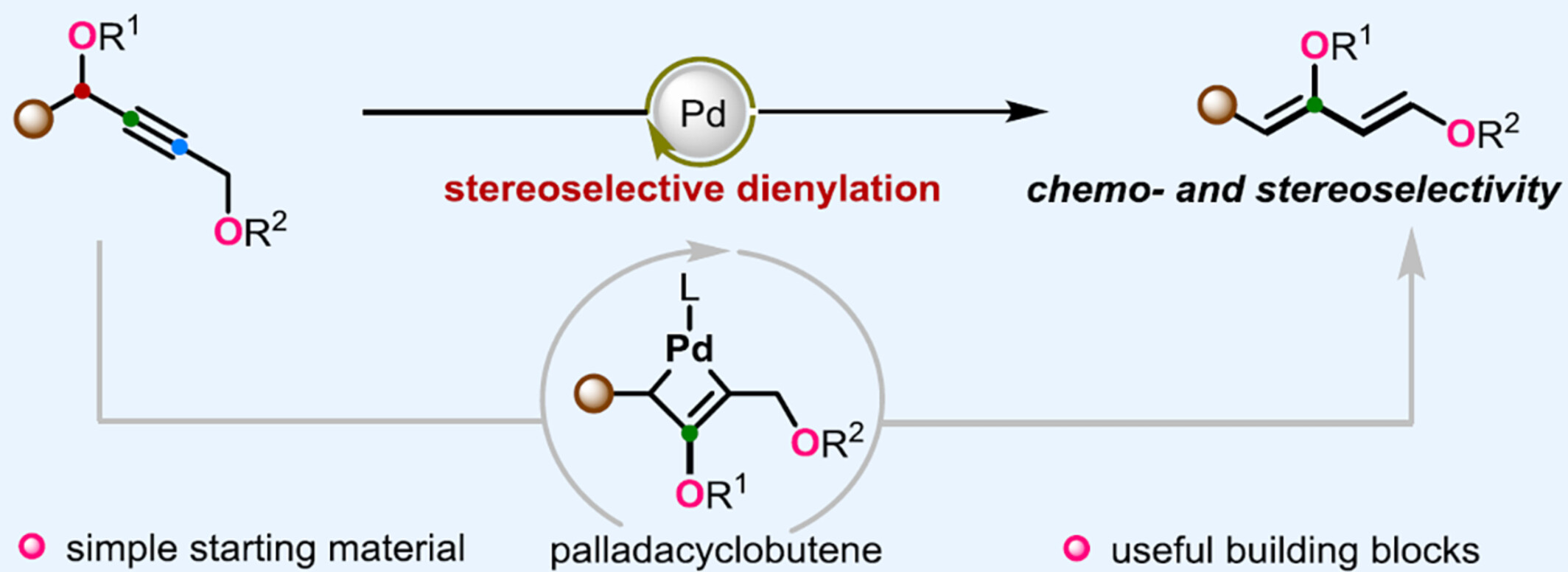
Pd-Catalyzed Dienylation of Propargylic Esters Enabling Highly Stereoselective Synthesis of Danishefsky-Type Trisubstituted Dienes
- Pages: 1993-1998
- First Published: 25 April 2024
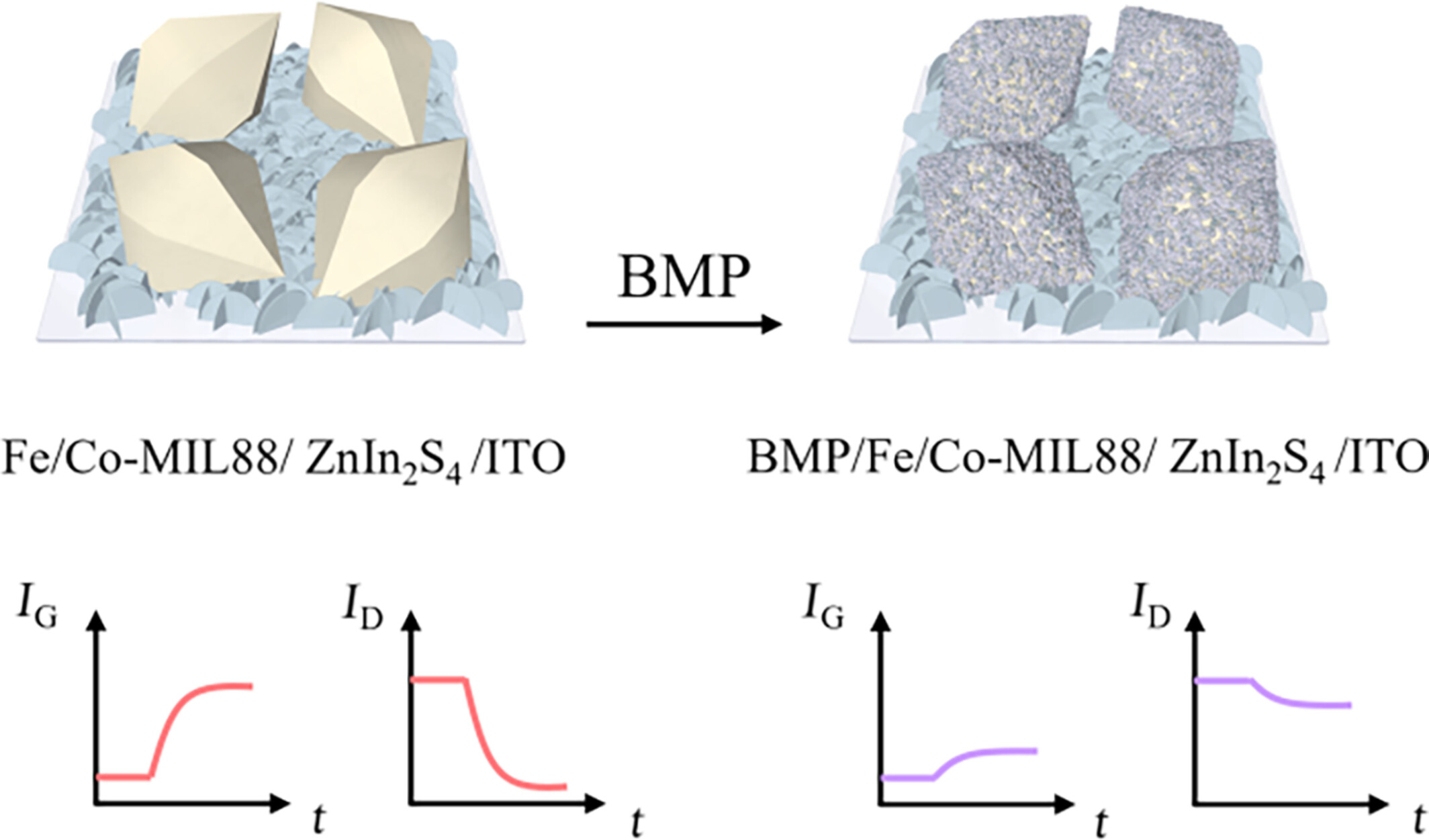
Metal-Organic Framework Nanozyme Enabling Dual-Functional Photo-Induced Charge Transfer and Biomimetic Precipitation for Advanced Organic Photoelectrochemical Transistor Bioanalysis
- Pages: 1999-2004
- First Published: 25 April 2024
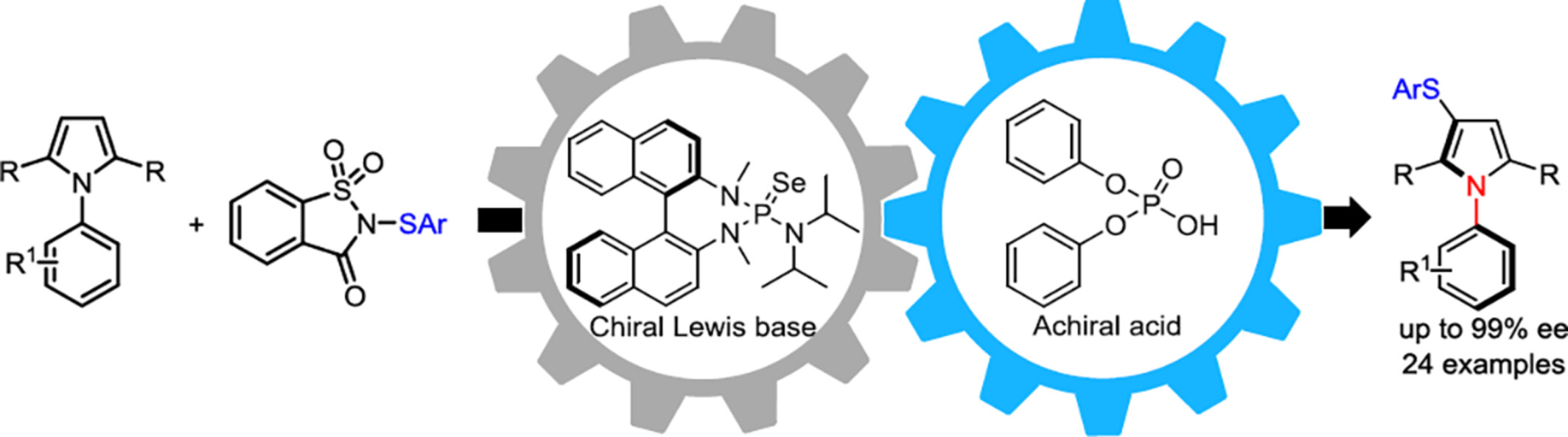
Chiral Lewis Base/Achiral Acid Co-Catalyzed Atroposelective Sulfenylation of Pyrrole Derivatives: Construction of C—N Axially Chiral Sulfides
- Pages: 2005-2009
- First Published: 25 April 2024
Generation and Applications of a Broad Atomic Oxygen Beam with a High Flux-Density via Collision-Induced Dissociation of O2
- Pages: 2010-2016
- First Published: 30 April 2024
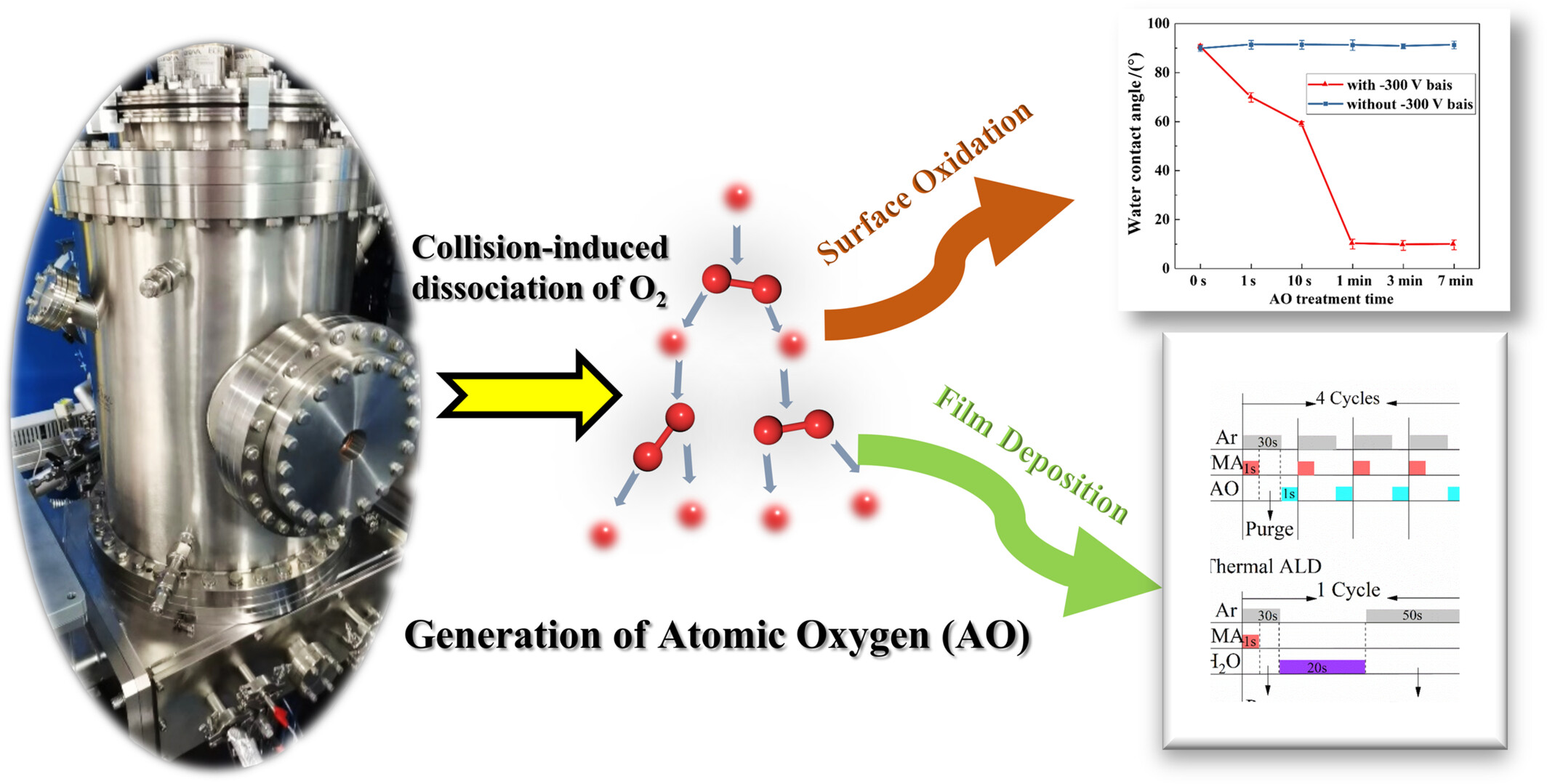
The report presented a method for generating a broad atomic oxygen (AO) beam with high flux-density. The feasibility of the method was verified through theoretical simulations. The device successfully generated high flux-density AO, which can facilitate the surface oxidation of materials. An AO-assisted atomic layer deposition process was employed to produce Al2O3 films that exhibited better performance.
Access to Enantioenriched Allylic Alcohols via Peptide-Mimic Phosphonium Salt-Catalyzed Asymmetric Aerobic Hydroxylation
- Pages: 2017-2022
- First Published: 29 April 2024
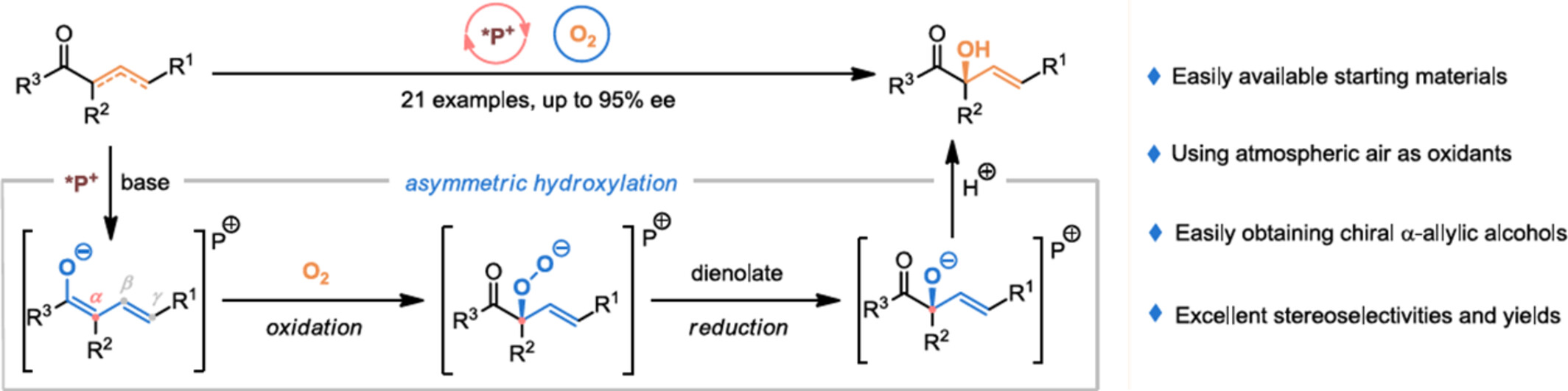
A peptide-mimic phosphonium salt-catalyzed asymmetric reductant-free aerobic hydroxylation of α,β-unsaturated and/or β,ϒ-unsaturated compounds has been developed, which provided modular and practical access to challenging enantioenriched allylic alcohols in high regio- and stereoselectivities and excellent yields. This protocol is characterized by the easy availability of starting materials, simple operation, and good functional group compatibility.
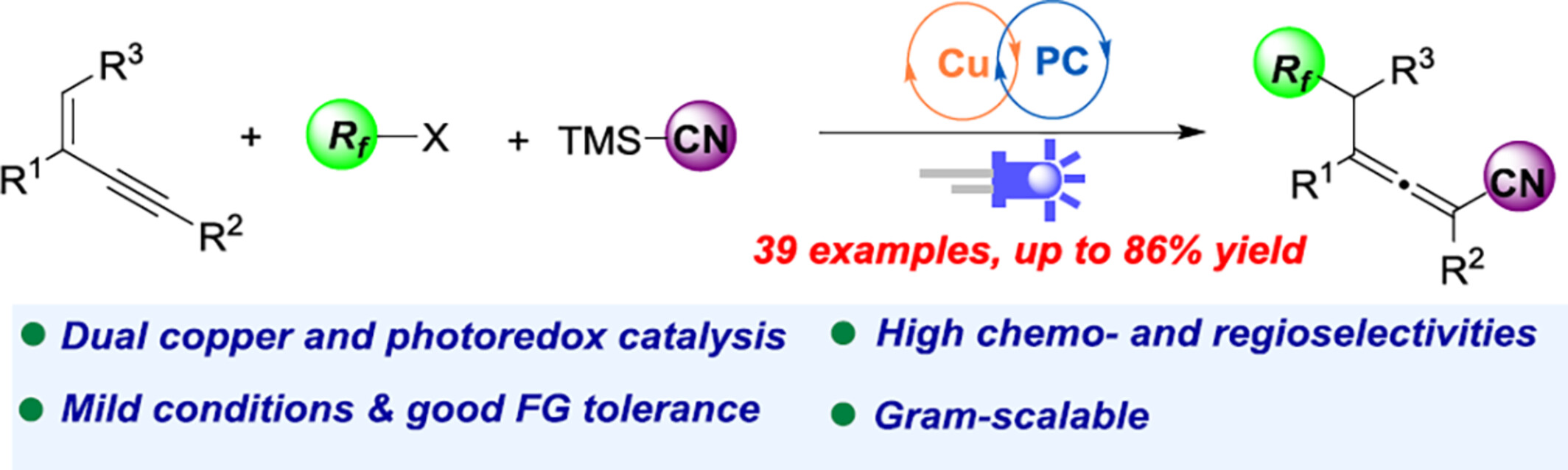
Copper/Photoredox-Catalyzed Regioselective 1,4-Addition to 1,3-Enynes: A Convenient Access to Perfluoroalkylated Allenes
- Pages: 2023-2028
- First Published: 30 April 2024
I2–DMSO-Mediated Multicomponent [2+1+1+1] Annulation Reaction via Ethyl Nitroacetate C—NO2 Bond Cleavage as a C1 Synthon: A Route to Multisubstituted β-Pyrrolidinones Derivatives with a Quaternary Center
- Pages: 2029-2034
- First Published: 29 April 2024
![I2–DMSO-Mediated Multicomponent [2+1+1+1] Annulation Reaction via Ethyl Nitroacetate C—NO2 Bond Cleavage as a C1 Synthon: A Route to Multisubstituted β-Pyrrolidinones Derivatives with a Quaternary Center](/cms/asset/4f621902-5485-4a74-b9bc-1622e6db550b/cjoc202400297-toc-0001-m.jpg)
A [2 + 1 + 1 + 1] cyclization reaction has been developed for the synthesis of multisubstituted β-pyrrolidinones from commercially available aryl methyl ketones, primary amines, and ethyl nitroacetate. In this I2–DMSO-meditated process, the C—NO2 bond of ethyl nitroacetate is cleaved, affording a C1 synthon, and the formation of two C—C and two C—N bonds and a quaternary carbon center are constructed in one pot. This method has good substrate compatibility and permits the late-stage modification of pharmaceutical compounds.
Ring-Opening Copolymerization of Biorenewable δ-Caprolactone with trans-Hexahydro-(4,5)-benzofuranone toward Closed-Loop Recyclable Copolyesters and Their Application as Pressure-Sensitive Adhesives
- Pages: 2035-2042
- First Published: 30 April 2024
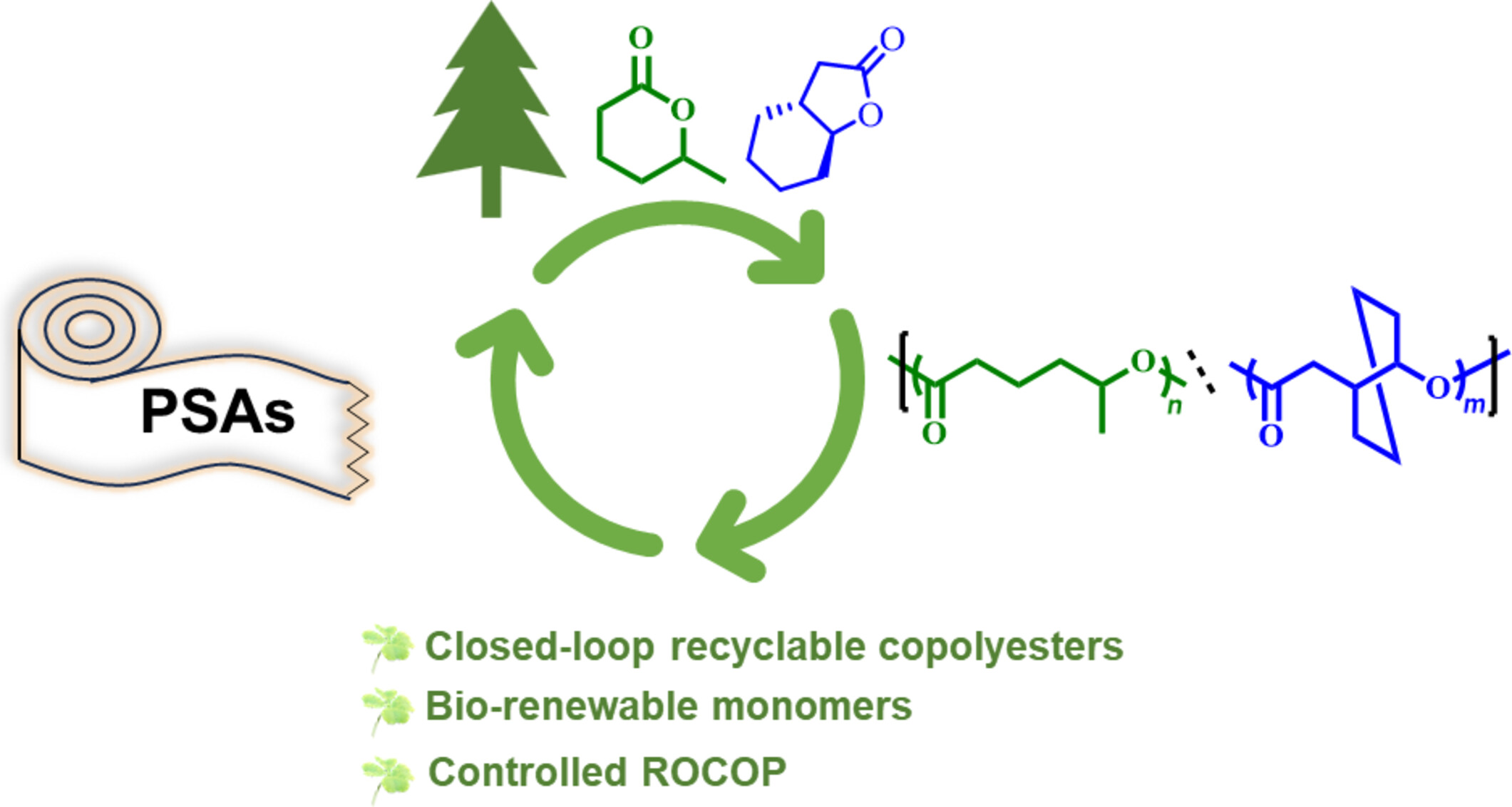
In this study, the bulk ring-opening copolymerization of bio-renewable δ-caprolactone and trans-hexahydro-(4,5)-benzofuranone was achieved to produce closed-loop recyclable copolyesters by using an organobase/urea binary catalyst at room temperature. The obtained copolyesters can be directly used as pressure sensitive adhesives, which exhibited comparable peel strength with the commercial PSA scotch tapes.
Magnetic Bistability in the Crystaline Fullerene Radical Salt
- Pages: 2043-2048
- First Published: 30 April 2024
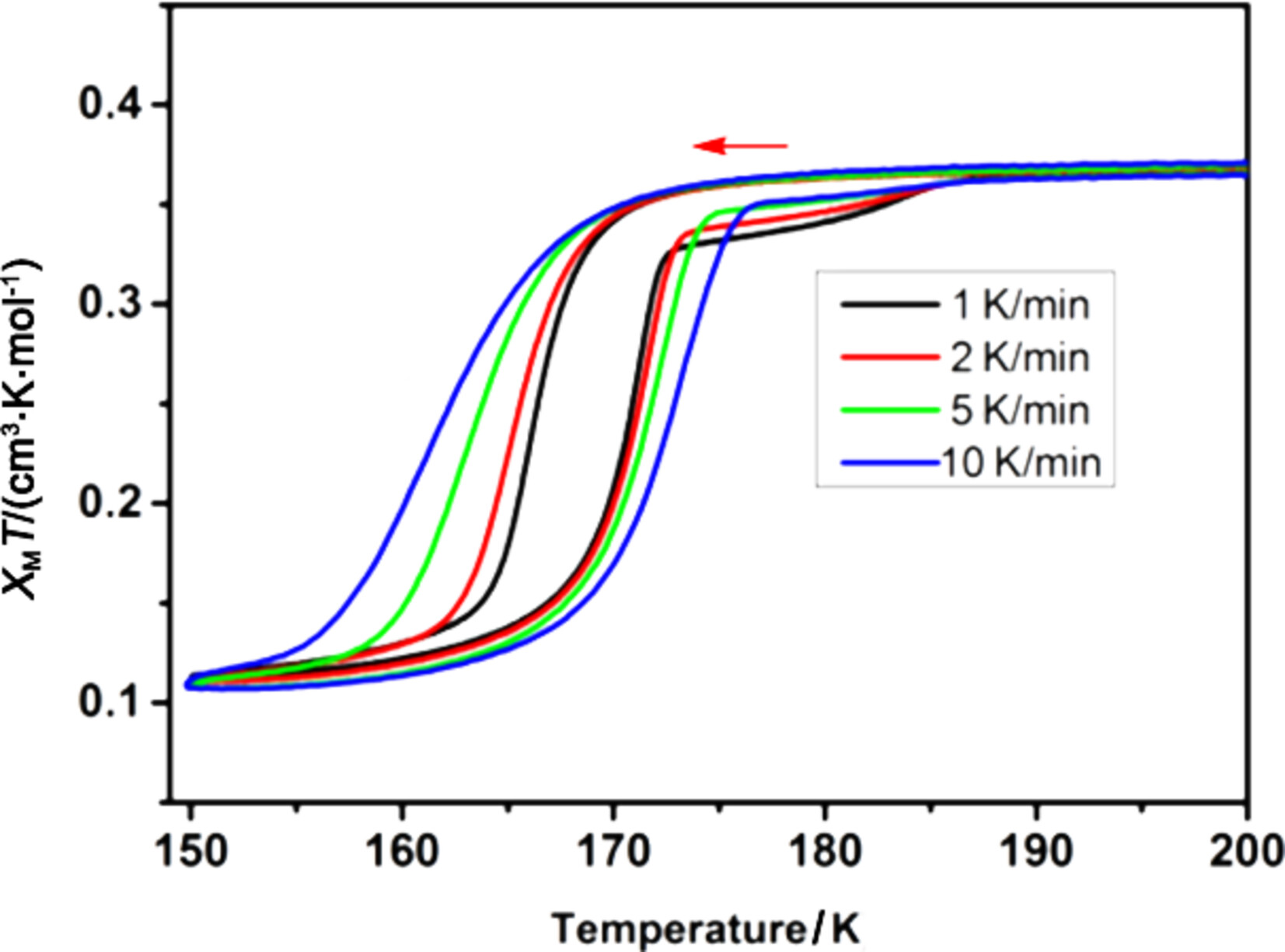
A fulleride based complex [Na(THF)5]C60 was isolated as single crystals, characterized by single crystal X-ray diffraction, EPR spectroscopy and SQUID measurement. The complex exhibits magnetic multistability with a large hysteresis loop, observed in the field of fullene chemistry for the first time. The magnetic multistability is associated with structural phase transitions.
Electrocatalytic Multicomponent Cascade Cross-Coupling for the Synthesis of Chalcogenosulfonates
- Pages: 2049-2055
- First Published: 07 May 2024
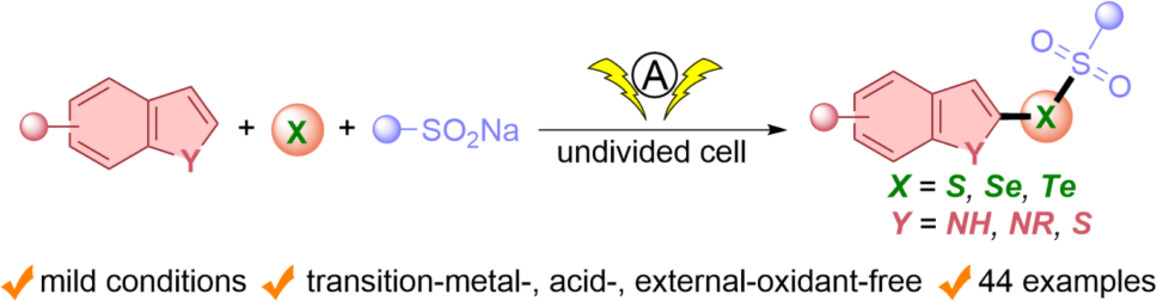
An electrocatalytic multicomponent cascade cross-coupling for the synthesis of chalcogenosulfonates under transition-metal-, acid- and external-oxidant-free conditions in an undivided cell has been developed. The robustness of this protocol has also been highlighted by successful late-stage modification of complex and widely used molecules.
Comprehensive Report
Efficient Chemical Prelithiation with Modificatory Li+ Solvation Structure Enabling Spatially Homogeneous SEI toward High Performance SiOx Anode
- Pages: 2056-2065
- First Published: 29 April 2024
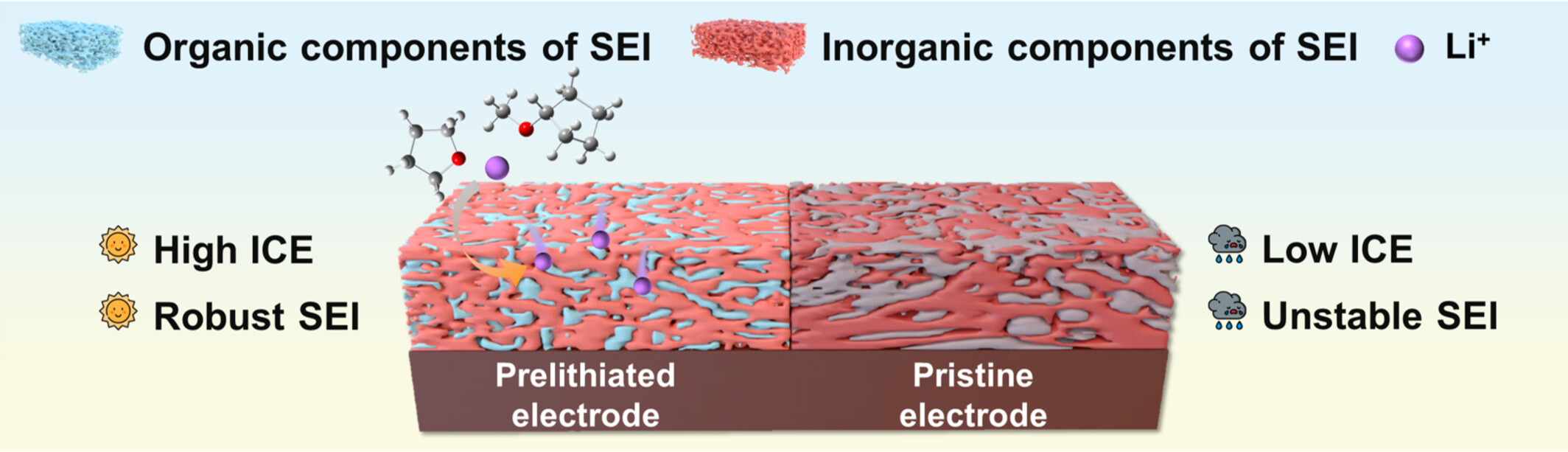
Weak solvent-driven prelithiation achieves efficient and uniform prelithiation effects, yielding LiF-rich, homogeneous, and stable solid electrolyte interphases without any alterations to the SiOx or electrolyte. The prelithiation process splits the SEI film formation process into two parts: pre-decomposition of the organic component and post-filling of the inorganic component, which broadens the idea of SEI film modulation strategy.
Emerging Topic
Emerging Bio-relevant Applications of Polymer Mechanochemistry
- Pages: 2066-2070
- First Published: 29 April 2024
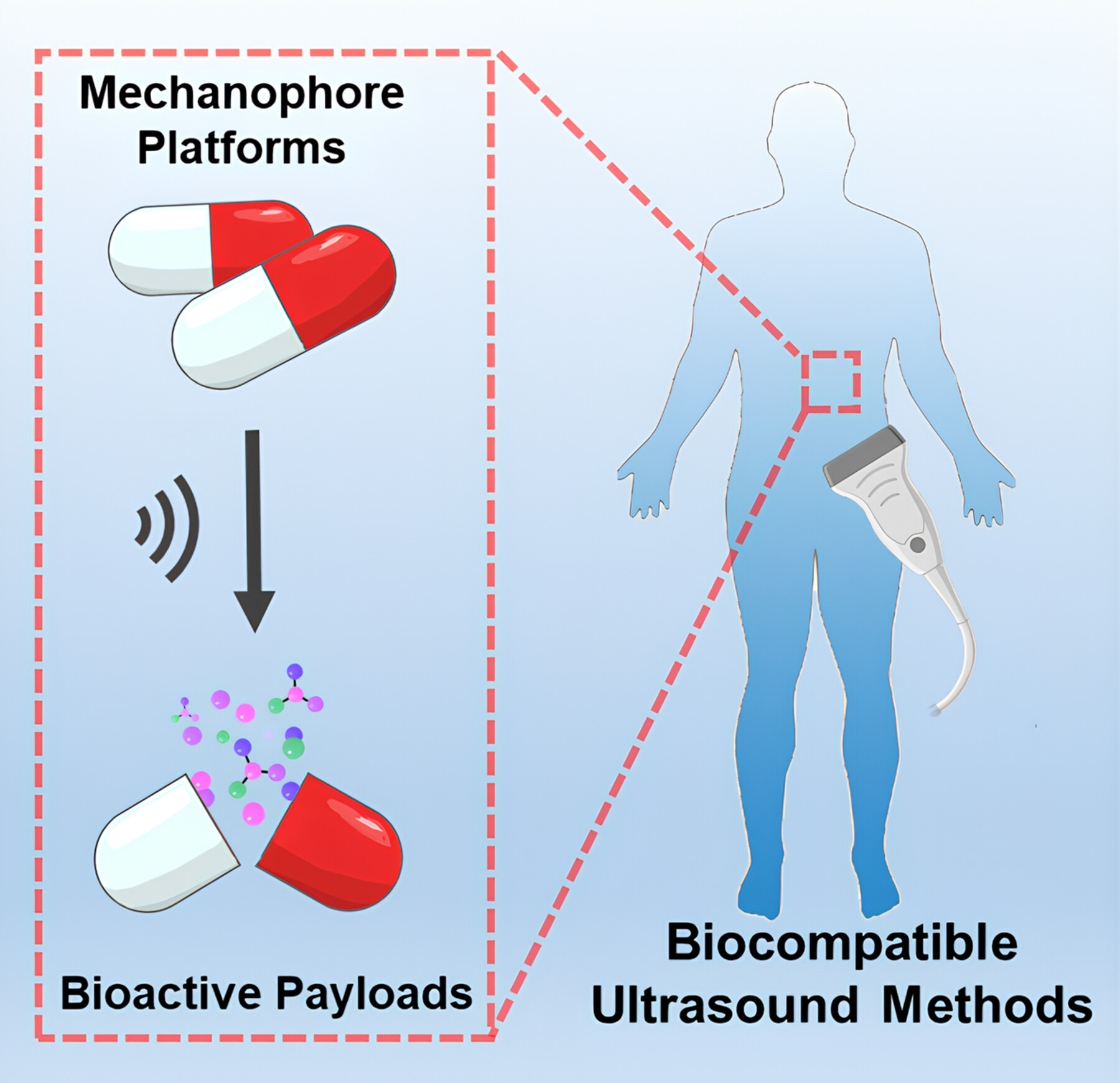
Recent efforts have been devoted to leveraging polymer mechanochemistry in bio-relevant applications. This perspective is aimed at providing a conceptual overview of how mechanophore platforms are developed to release bioactive payloads and exploring biocompatible activation strategies for potential biomedical and clinical usage. The potential and challenges in relevant fields are discussed at the end.
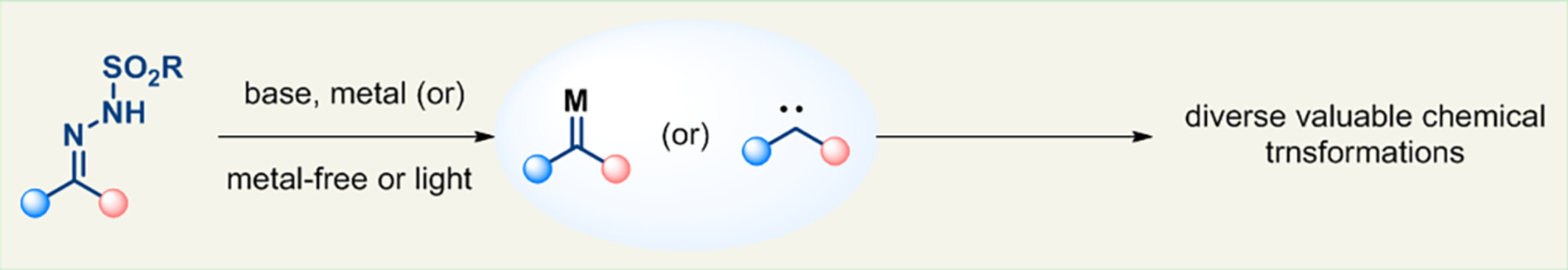
Critical Review
Who is Who in the Carbene Chemistry of N-Sulfonyl Hydrazones
- Pages: 2071-2108
- First Published: 27 May 2024
Meet Our New Editorial Board Members of Spotlights
Meet Our New Editorial Board Members of Spotlights
- Pages: 2109-2114
- First Published: 01 August 2024
Inside Back Cover
Inside Back Cover
- Page: 2119
- First Published: 01 August 2024
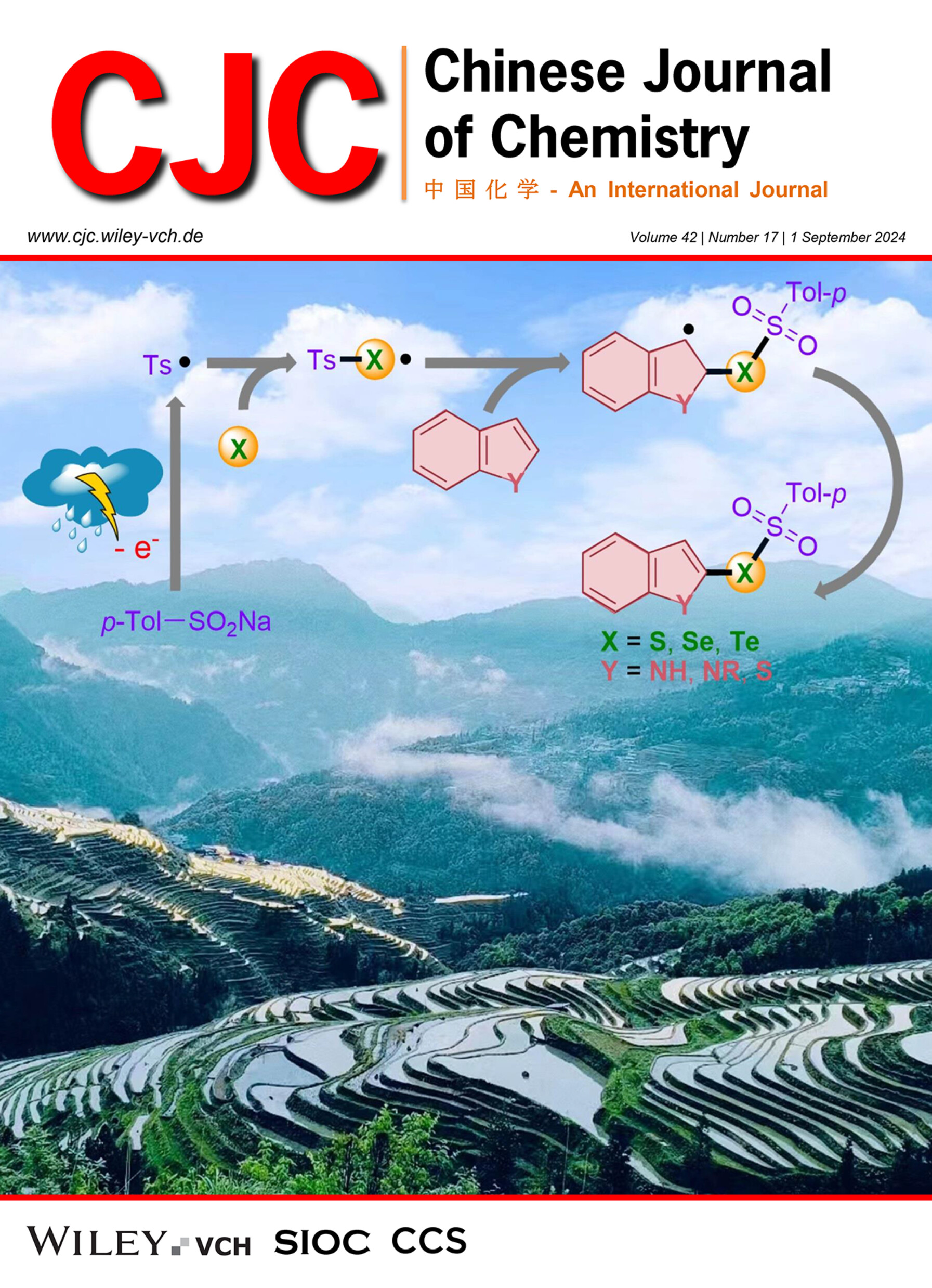
An electrocatalytic multicomponent cascade cross-coupling for the synthesis of chalcogenosulfonates from indoles, sulfinic acid sodium salts and the simple chalcogen has been established. This approach does not require the use of transition metals, acids, and external oxidants. More details are discussed in the article by Gu et al. on page 2049—2055.
Back Cover
Back Cover
- Page: 2120
- First Published: 01 August 2024

Chemical prelithiation based on prelithiation reagents is the efficient method to compensate for active Li+ and improve electrode ICE and energy density of SiOx anode. In the efficient prelithiation reagent driven by the weak solvent cyclopentyl methyl ether (CPME), the lithium-biphenyl compound carries Li+ and transfers it to the interior of the active material to achieve the aforementioned purpose. The entire process is analogous to “dragonfly skimming the surface of the water”, whereby the dragonfly's tail contacts the surface of the water and discharges the dragonfly eggs into the water, thus completing the reproductive behaviour. Lithium-biphenyl compounds act in the manner analogous to that of dragonflies, facilitating the transfer of Li+ to the active materials, thereby completing the prelithiation process. More details are discussed in the article by Wu et al. on page 2056—2065.