Chiral Lewis Base/Achiral Acid Co-Catalyzed Atroposelective Sulfenylation of Pyrrole Derivatives: Construction of C—N Axially Chiral Sulfides
Qin Yang
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorHui-Yun Luo
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorDeng Zhu
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorXin-Yu Zhang
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Hua Ke
Engineering Technology Research Center for Environmental Protection Materials, Pingxiang University, Pingxiang, Jiangxi, 337055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhi-Min Chen
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorQin Yang
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorHui-Yun Luo
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorDeng Zhu
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorXin-Yu Zhang
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
Search for more papers by this authorCorresponding Author
Hua Ke
Engineering Technology Research Center for Environmental Protection Materials, Pingxiang University, Pingxiang, Jiangxi, 337055 China
E-mail: [email protected]; [email protected]Search for more papers by this authorCorresponding Author
Zhi-Min Chen
School of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai, 200240 China
State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou, Gansu, 730000 China
E-mail: [email protected]; [email protected]Search for more papers by this authorComprehensive Summary
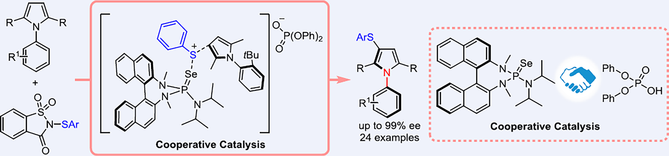
Chiral BINAM-derived selenide/achiral acid co-catalyzed atroposelective electrophilic sulfenylation of pyrrole derivatives has been realized for the first time. A variety of C—N axially chiral sulfur-containing pyrrole derivatives were readily obtained in moderate to good yields with moderate to excellent enantioselectivities. This catalytic system involves sequential desymmetrization and kinetic resolution.
Supporting Information
| Filename | Description |
|---|---|
| cjoc202400255_sup_0001_supinfo.pdfPDF document, 9.8 MB |
Appendix S1: Supporting Information |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Selected example: Faigl, F.; Erdélyi, Z.; Holczbauer, T.; Mátravölgyi, B. Highly Efficient Stereoconservative Syntheses of New, Bifunctional Atropisomeric Organocatalysts. Arkivoc 2016, (iii), 242−261.
10.3998/ark.5550190.p009.505 Google Scholar
- 2Selected example: Hughes, C.-C.; Prieto-Davo, A.; Jensen, P.-R.; Fenical, W. The Marinopyrroles, Antibiotics of an Unprecedented Structure Class from a Marine Streptomyces sp. Org. Lett. 2008, 10, 629−631.
- 3Selected recent reviews: (a) Zhang, H.-H.; Shi, F. Organocatalytic Atroposelective Synthesis of Indole Derivatives Bearing Axial Chirality: Strategies and Applications. Acc. Chem. Res. 2022, 55, 2562−2580; (b) Cheng, J.; Xiang, S.-H.; Tan, B. Organocatalytic Enantioselective Synthesis of Axially Chiral Molecules: Development of Strategies and Skeletons. Acc. Chem. Res. 2022, 55, 2920−2937; (c) Chen, W.; Zhong, X.; Xing, J.; Wu, C.; Gao, Y. Progress in Asymmetric Catalytic Synthesis of C−N Axis Chiral Compounds. Chin. J. Org. Chem. 2024, 44, 349−377; Selected recent examples: (d) Zhang, L.; Zhang, J.; Ma, J.; Cheng, D.-J.; Tan, B. Highly Atroposelective Synthesis of Arylpyrroles by Catalytic Asymmetric Paal-Knorr Reaction. J. Am. Chem. Soc. 2017, 139, 1714−1717; (e) Lu, S.; Lovato, K.; Ong, J.-Y.; Poh, S.-B.; Ng, X.-Q.; Kürti, L.; Zhao, Y. Practical Access to Axially Chiral Sulfonamides and Biaryl Amino Phenols via Organocatalytic Atroposelective N-alkylation. Nat. Commun. 2019, 10, 3061−3070; (f) Zhang, S.; Yao, Q.-J.; Liao, G.; Li, X.; Li, H.; Chen, H.-M.; Hong, X.; Shi, B.-F. Enantioselective Synthesis of Atropisomers Featuring Pentatomic Heteroaromatics by Pd-Catalyzed C−H Alkynylation. ACS Catal. 2019, 9, 1956−1961; (g) Ye, C.-X.; Chen, S.; Han, F.; Xie, X.; Ivlev, S.; Houk, K. N.; Meggers, E. Atroposelective Synthesis of Axially Chiral N-Arylpyrroles by Chiral-at-Rhodium Catalysis. Angew. Chem. Int. Ed. 2020, 59, 13552−13556; (h) Zhao, Y.; Liu, N.-N.; Zhong, S.-P.; Wen, Z.-W.; Tao, W. A Central-to- Axial Chirality Conversion Strategy for the Synthesis of C-N Axially Chiral N-Arylpyrroles. Org. Lett. 2022, 24, 2842−2846; (i) Sheng, F.-T.; Yang, S.; Wu, S.-F.; Zhang, Y.-C.; Shi, F. Catalytic Asymmetric Synthesis of Axially Chiral 3,3'-Bisindoles by Direct Coupling of Indole Rings. Chin. J. Chem. 2022, 40, 2151−2160; (j) Huang, Q.-Q.; Wu, S.-F.; Yang, S.; Wang, X.; Zhong, Z.; Zhang, Y.-C.; Shi, F. Design and catalytic atroposelective synthesis of axially chiral isochromenone-indoles. Sci. China Chem. 2022, 65, 1929−1937; (k) Zhang, P.; Guo, C.-Q.; Yao, W.; Lu, C.-J.; Li, Y.-Z.; Paton, R.; Liu, R.-R. Pd-Catalyzed Asymmetric Amination of Enamines: Expedient Synthesis of Structurally Diverse N-C Atropisomers. ACS Catal. 2023, 13, 7680−7690; (l) Jin, L.; Shi, B. Design and Catalytic Asymmetric Synthesis of Furan-Indole Compounds Bearing Both Axial and Central Chirality. Chin. J. Org. Chem. 2024, 44, 657−659.
- 4 Zhang, L.; Xiang, S.-H.; Wang, J.; Xiao, J.; Wang, J.-Q.; Tan, B. Phosphoric Acid-Catalyzed Atroposelective Construction of Axially Chiral Arylpyrroles. Nat. Commun. 2019, 10, 566−575.
- 5Selected recent reviews: (a) Matviitsuk, A.; Panger, J.-L.; Denmark, S. E. Catalytic, Enantioselective Sulfenofunctionalization of Alkenes: Development and Recent Advances. Angew. Chem. Int. Ed. 2020, 59, 19796−19819; (b) Liao, L.; Zhao, X. Indane-Based Chiral Aryl Chalcogenide Catalysts: Development and Applications in Asymmetric Electrophilic Reactions. Acc. Chem. Res. 2022, 55, 2439−2453; (c) Zhu, D.; Chen, Z.-M. Application of Chiral Lewis Base/Brønsted Acid Synergistic Catalysis System in Enantioselective Synthesis of Organic Sulfides. Chin. J. Org. Chem. 2022, 42, 3015−3032; (d) Cao, R.-F.; Chen, Z.-M. Catalytic asymmetric synthesis of sulfur-containing atropisomers by C−S bond formations. Sci. China Chem. 2023, 66, 3331–3346.
- 6(a) Luo, H.-Y.; Li, Z.-H.; Zhu, D.; Yang, Q.; Cao, R.-F.; Ding, T.-M.; Chen, Z.-M. Chiral Selenide/Achiral Sulfonic Acid Cocatalyzed Atroposelective Sulfenylation of Biaryl Phenols via a Desymmetrization/Kinetic Resolution Sequence. J. Am. Chem. Soc. 2022, 144, 2943−2952; (b) Zhang, X.-Y.; Zhu, D.; Huo, Y.-X.; Chen, L.-L.; Chen, Z.-M. Atroposelective Sulfenylation of Biaryl Anilines Catalyzed by Chiral SPINOL-Derived Selenide. Org. Lett. 2023, 25, 3445−3450; (c) Zhu, D.; Yu, L.; Luo, H.-Y.; Xue, X.-S.; Chen, Z.-M. Atroposelective Electrophilic Sulfenylation of N-Aryl Aminoquinone Derivatives Catalyzed by Chiral SPINOL-Derived Sulfide. Angew. Chem. Int. Ed. 2022, 61, e202211782; (d) Zhu, D.; Mu, T.; Li, Z.-L.; Luo, H.-Y.; Cao, R.-F.; Xue. X.-S.; Chen, Z.-M. Enantioselective Synthesis of Planar-Chiral Sulfur-Containing Cyclophanes by Chiral Sulfide Catalyzed Electrophilic Sulfenylation of Arenes. Angew. Chem. Int. Ed. 2024, 63, e202318625.
- 7Selected examples: (a) Xie, Y.-Y.; Chen, Z.-M.; Luo, H.-Y.; Shao, H.; Tu, Y.-Q.; Bao, X.; Cao, R.-F.; Zhang, S.-Y.; Tian, J.-M. Lewis Base/Brønsted Acid Co-catalyzed Enantioselective Sulfenylation/Semipinacol Rearrangement of Di- and Trisubstituted Allylic Alcohols. Angew. Chem. Int. Ed. 2019, 58, 12491−12496; (b) Liu, X.-D.; Luo, Y.; Huo, X.; Luo, H.-Y.; Cao, R.-F.; Chen, Z.-M. Chiral Sulfide/Phosphoric Acid Cocatalyzed Enantioselective Intermolecular Oxysulfenylation of Alkenes with Phenol and Alcohol O-Nucleophiles. CCS Chem. 2022, 4, 3342−3354; (c) Liu, X.-D.; Ye, A-H.; Chen, Z.-M. Catalytic Enantioselective Intermolecular Three-Component Sulfenylative Difunctionalizations of 1,3-Dienes. ACS Catal. 2023, 13, 2715−2722; (d) Chen, L.-L.; Cao, R.-F.; Ke, H.; Chen, Z.-M. Lewis Base Catalyzed Selenofunctionalization of Alkynes with Acid-controlled Divergent Chemoselectivity. Chin. J. Chem. 2024, 42, 1623−1629.
- 8CCDC 2280030 (compound 3a) contains the supplementary crystallographic data for this paper. The data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing [email protected], or by contacting The Cam-bridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.




